Abstract
Shigella species cause bacillary dysentery in humans by invasion, intracellular multiplication, spread to adjacent cells, and induction of brisk inflammatory responses in the intestinal epithelium. In vitro data suggest that lactoferrin, a glycoprotein present in human mucosal secretions, has a role in protection from bacterial enteric infections. We sought to determine the activity of lactoferrin in vivo, using the concentration present in human colostrum, to investigate its effect on the development of clinical and pathological evidence of inflammation in a rabbit model of enteritis. Lactoferrin protected rabbits infected with Shigella flexneri from developing inflammatory intestinal disease. Typical histological changes in ill animals included villous blunting with sloughing of epithelial cells, submucosal edema, infiltration of leukocytes, venous congestion, and hemorrhage. Lactoferrin at a concentration normally found in human colostrum blocks development of S. flexneri-induced inflammatory enteritis.
Dysentery due to Shigella spp. is among the most communicable and severe forms of bacterial gastroenteritis in humans (43). The virulence of Shigella is due to its ability to invade, multiply, spread intracellularly, and induce inflammation within the intestinal epithelium (33). Most of the genes responsible for invasion of eukaryotic cells are located on a 230-kb virulence plasmid (1). Entry into mammalian cells is regulated by a plasmid-encoded type III secretory system located on a 31-kb locus that codes for the invasion plasmid antigens IpaA, IpaB, IpaC, and IpaD. This secretory mechanism is activated upon contact with epithelial cells (49). Upon entry into host cells, bacteria gain access to the cytoplasm by lysing the phagocytic vacuole and rapidly multiplying and spreading (26, 32, 41)
Epidemiologic studies have shown that breast-feeding decreases the severity of Shigella sp. infection in infants who become colonized early in life (2, 12, 13, 20, 22, 25). Immune and nonimmune components of milk may be relevant to this protection. Among the nonantibody factors is lactoferrin, an iron-binding glycoprotein of 78 kDa that is resistant to proteolytic enzymes (10). However, epidemiologic data cannot separate the effect of lactoferrin from those of other potentially protective human milk factors. Lactoferrin, antilipopolysaccharide (anti-LPS) and anti-invasion plasmid antigen secretory immunoglobulin A (IgA), lysozyme, and oligosaccharides may each play a role in protection of infants from shigellosis. Although multiple effects of lactoferrin have been demonstrated in vitro, there are no animal model studies demonstrating a role in the gut in vivo. The objective of these studies was to determine whether lactoferrin at concentrations found in human colostrum (0.125 mM) could prevent the development of clinical and pathological changes in an in vivo rabbit model of inflammatory enteritis.
MATERIALS AND METHODS
Lactoferrin was obtained from Agennix Inc. Lactoferrin is expressed in Aspergillus awamori as a glucoamylase fusion polypeptide secreted into the medium and processed to mature human lactoferrin by an endogenous KEX-2 peptidase. The recombinant protein retains full biological activity (47).
Rabbit infection model.
Four-week-old New Zealand White rabbits were challenged with 108 CFU of log-phase Shigella flexneri serotype 5 strain M90T. Preliminary experiments were done with 107 to 5 × 109 CFU before selecting a dose of 108 CFU. S. flexneri 5 strain M90T, stored in charcoal-yeast extract glycerol at −70°C, was grown overnight at 37°C on Congo Red agar to verify the presence of virulence genes. A log-phase culture in brain heart infusion broth (BHI) was centrifuged, washed with 10 mM phosphate-buffered saline (PBS), pH 7.4, and incubated with end-over-end rotation at 37°C for 1 h in PBS in the absence or presence of lactoferrin (0.125 mM). The bacteria were then centrifuged, washed, and resuspended in PBS. The number of bacteria was estimated from a curve relating optical density to CFU of a log-phase culture prior to inoculation into the rabbits. The effect of lactoferrin treatment on bacterial viability was assessed by incubating inocula of lactoferrin-treated or PBS-treated washed organisms, adding them to BHI, and performing serial determinations each hour for the next 3 h of incubation at 37°C.
For rabbit infection the bacteria were delivered in 5 ml of buffer. With each group of animals studied (usually six per day) virulence of the S. flexneri was confirmed by Congo Red uptake of the organisms used for inoculation of animals. Lactoferrin was used in a physiologic concentration (0.125 mM). The inoculum was given by orogastric tube to anesthetized rabbits after pretreatment with 7.5 mg of ranitidine to block acid secretion and two 5-ml doses of NaHCO3 (5%) followed by 0.2 mg of loperamide. This method is a modification of a previously described procedure (20). The rabbits were then given ad lib access to PBS with or without lactoferrin (0.125 mM) until sacrifice; the continuation of lactoferrin was meant to simulate the situation that occurs during feeding of human infants. Twelve animals were monitored for 15 days to determine the course of infection; weight and body temperature were determined daily. An additional 68 animals (35 given lactoferrin and 33 given just buffer) were sacrificed after 24 h so that the intestines could be examined grossly and microscopically for evidence of inflammatory enteritis. Macroscopically, inflammation was assessed by presence of edema, erythema, and hemorrhage. For microscopic examination, the most distal ileal Peyer's patch and a 1-cm2 area on either side of it were fixed in formalin, stained with hematoxylin and eosin, and evaluated by a blinded observer. Each slide was scored for submucosal edema, submucosal hemorrhage, venous congestion, inflammatory submucosal leukocyte infiltrate, and shortening of villi (with one point given for the presence of each finding and zero points given for the absence of each finding; maximum score, 5). The studies described in this report were all reviewed and approved by the Animal Welfare Committee of the University of Texas Health Science Center at Houston.
Statistical analysis.
Parametric data were analyzed with the Student t test. The chi-square test was used for analyzing differences in proportions. Two-tailed tests were used.
RESULTS
Effect of lactoferrin on bacterial viability.
Bacteria that had been previously incubated for 1 h at 37°C in lactoferrin, washed, and cultured had a number CFU essentially identical to that observed for those cultures that had been preincubated in PBS. At 1, 2, and 3 h after initiation of culture in BHI, the lactoferrin-treated organism CFU were 2.9 × 108, 4.4 × 108, and 6.2 × 108 compared to the organism that had been incubated in buffer only prior to culture, whose CFU were 2.8 × 108, 4.4 × 108, and 6.8 × 108, respectively. The data are geometric means for seven separate experiments. There is no significant difference between the growth curves.
Clinical status.
Twelve animals monitored for 2 weeks after Shigella inoculation all survived. Diarrhea did not develop, perhaps reflecting the loperamide used to slow intestinal motility and promote development of infection. There were no significant differences in their weights during the 2 weeks. The only significant difference was that the animals not given lactoferrin had a significantly higher body temperature [39.9 ± 0.2°C (mean ± standard error of the mean) versus 38.9 ± 0.2°C, P < 0.01) 24 h after inoculation. Subsequent temperature measurements were not significantly different. Adult New Zealand White rabbit body temperature range is normally 38.9 to 39.6°C (29). The animals not given lactoferrin developed ruffled fur and were less active during the initial febrile period.
Macroscopic changes of enteritis.
Groups of animals were sacrificed 24 h after inoculation in order to look for evidence of enteritis. Gross evidence of inflammatory changes (Fig. 1) developed significantly more often in rabbits infected without lactoferrin treatment (22 of 33 [67%]) than in those infected with lactoferrin treatment (4 of 35 [11%]) (P < 0.001).
FIG. 1.
Macroscopic evidence of severe inflammatory enteritis in a portion of ileum of a rabbit infected with Shigella and not receiving lactoferrin treatment (bottom). Normal distal ileum in an infected animal treated with lactoferrin (top).
Microscopic evidence of inflammatory changes.
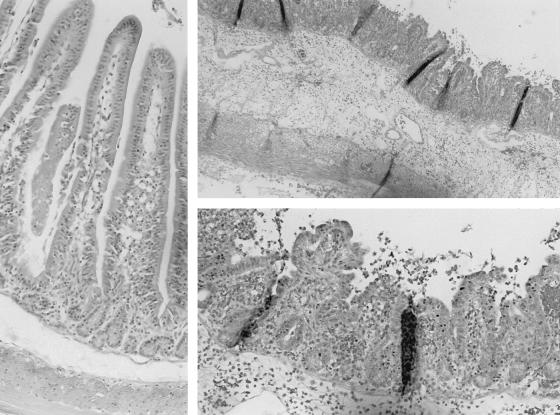
Fig. 2 shows the histology of an infected rabbit given lactoferrin and an infected animal not given lactoferrin. Typical histological changes in animals not given lactoferrin included villous blunting with sloughing of epithelial cells, submucosal edema, infiltration of leukocytes, venous congestion, and hemorrhage (Fig. 2). The degree of inflammation interpreted by a blinded observer was significantly less in the infected animals treated with lactoferrin than in those not given lactoferrin [inflammation score, 2.1 ± 0.3 versus 3.8 ± 0.3 (mean ± standard error of the mean); P < 0.001].
FIG. 2.
The bottom left panel shows the normal histological appearance of a section of ileum of a rabbit infected with Shigella and receiving lactoferrin treatment. (score, 0) The other panels show typical histological changes in a section of ileum of ill rabbits infected with Shigella and not receiving lactoferrin treatment (right top, low-power magnification; bottom, high-power magnification) (score 5) showing blunting of villi, submucosal edema, hemorrhages, and severe inflammation.
DISCUSSION
There are many activities that have been attributed to lactoferrin based on in vitro studies. Lactoferrin has been thought to protect against gram-negative bacteria in a variety of ways. Lactoferrin has both bacteriostatic (37, 39, 44) and bactericidal activity (4, 5, 16). It chelates iron required for bacterial growth. In iron-deficient media, this results in bacteriostasis. However, the antibacterial activity of lactoferrin is not due solely to its iron-binding capacity. A pepsin-derived fragment of lactoferrin, lactoferricin, has iron-independent bactericidal activity that is associated with release of LPS (17, 18, 51). An 11-residue peptide (FQWQRNMRKVR) is responsible for the bactericidal activity (6). Lactoferrin binds to the phosphate group of the lipid A moiety of LPS (3, 9). There are both high-affinity and low-affinity LPS binding sites in lactoferrin (14). Lactoferrin also binds to outer membrane proteins (porins) of Escherichia coli and Salmonella and Shigella species, thereby destabilizing the bacterial exterior surface (19, 34, 38, 46). Tissue culture models suggest that lactoferrin inhibits adhesion of enterotoxigenic E. coli, enteropathogenic E. coli, diffusely adherent E. coli, and enteroaggregative E. coli (27, 35, 36). Lactoferrin also blocks hemagglutination caused by enterotoxigenic E. coli (23). However, there is little proof that these in vitro observations regarding diarrheagenic E. coli have any relevance for these or other enteropathogens in a living animal.
There are few in vivo demonstrations of lactoferrin's relevance. Oral lactoferrin reduces E. coli urinary tract infection in mice (24), and intravenous lactoferrin improves survival during E. coli sepsis in mice (52). It is generally assumed that human milk protects from bacterial intestinal infection in part due to lactoferrin, but there are no animal model studies evaluating its effect in isolation. It is unclear whether lactoferrin protects rabbits from inflammatory enteritis by one of the above previously described mechanisms or some other process. A remarkable feature of the studies of Shigella-induced enteritis is the decrease in inflammation noted in the lactoferrin-treated rabbits. Lactoferrin has been noted to have anti-inflammatory effects in several model systems. (7, 28, 52) The decrease in intestinal inflammatory findings we observed might have been due to direct effects of lactoferrin either on the bacteria or on the gut. Data strongly suggest that the anti-inflammatory effect of lactoferrin is due to its binding lipid A (3) and soluble CD14. By preventing LPS binding to LPS binding protein and membrane CD14 (15), lactoferrin decreases secretion of multiple cytokines (interleukin-6 [IL-6], IL-1, and tumor necrosis factor alpha) by macrophages (13, 30, 31). However, this mechanism is very unlikely to be responsible for the effects we observed in this rabbit model. The intestinal macrophages of rabbits (50), like those of humans (42), lack CD14 so that a role for lactoferrin-mediated blockade of LPS-induced inflammation in shigellosis seems untenable. Indeed, the lack of CD14 receptors on gut macrophages is probably an important factor in the lack of inflammation in normal intestine despite massive exposure to LPS. The data currently suggest that although LPS may contribute to the inflammatory response in shigellosis (8), it is not the primary inflammatory stimulus. Shigella spp. induce inflammation via effects of IpaB on IL-1β-converting enzyme, not via LPS release and LPS binding protein/membrane CD14-mediated cytokine induction. Shigella spp. are taken up by M cells (40, 48) and infect Peyer's patch macrophages where IpaB binds to interleukin-1β-converting enzyme (11, 45). Apoptosis of macrophages, T cells, and B cells then occurs (54-56). Release of IL-1α and IL-1β evokes an inflammatory reaction that causes polymorphonuclear leukocytes to migrate through the epithelium into the lumen, disrupting the epithelium and allowing massive entry of bacteria into the submucosa with further tissue destruction (53). Organisms taken up at Peyer's patches should have been able to induce a local inflammatory reaction if IpaB were present. The absence of inflammation therefore suggested that IpaB had been affected by lactoferrin treatment. Lactoferrin decreases invasiveness of S. flexneri (21). Lactoferrin causes loss and degradation of invasion plasmid antigens (21a).
Although it is not clear which of the biologic activities of lactoferrin is responsible for protection, these studies clearly show that at the intact gut level, lactoferrin protects against development of inflammatory enteritis. These data suggest that if the human gut behaves like that of the rabbit, lactoferrin may play an important role in protection of breast fed infants from bacillary dysentery and may merit study as a potential agent for therapy of Shigella-induced inflammatory enteritis.
Acknowledgments
This work was supported in part by PHS award PO1-HD13021 and in part by Agennix Corporation of Houston, Tex.
Editor: J. D. Clements
REFERENCES
- 1.Adler, B., C. Sasakawa, T. Tobe, S. Makino, K. Komatsu, and M. A. Yoshikawa. 1989. Dual transcriptional activation system for the 230 kb plasmid genes coding for virulence-associated antigens of Shigella flexneri. Mol. Microbiol. 3:627-635. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, F., J. D. Clemens, M. R. Rao, D. A. Sack, M. R. Khan, and E. Haque. 1992. Community based evaluation of the effect of breast feeding on the risk of microbiologically confirmed or clinically presumptive shigellosis in Bangladeshi children. Pediatrics 90:406-411. [PubMed] [Google Scholar]
- 3.Appelmelk, B. J., Y. Q. An, M. Geerts, B. G. Thijs, H. A. de Boer, D. M. MacLaren, J. de Graaff, and J. F. Nuijens. 1994. Lactoferrin is a lipid A binding protein. Infect. Immun. 62:2628-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold, R. R., M. Brewer, and J. J. Gauthier. 1980. Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect. Immun. 28:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold, R. R., M. F. Cole, and J. R. McGhee. 1977. A bactericidal effect for human lactoferrin. Science 197:263-265. [DOI] [PubMed] [Google Scholar]
- 6.Azuma, M., T. Jojima, I. Yokoyama, H. Tajiri, K. Yoshikawa, S. Saga, and C. A. Del Carpio. 1999. Antibacterial activity of multiple antigen peptides homologous to a loop region in human lactoferrin. J. Peptide Res. 54:237-241. [DOI] [PubMed] [Google Scholar]
- 7.Baveye, S., E. Elass, D. G. Fernig, C. Blanquart, J. Mazurier, and D. Legrand. 2000. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect. Immun. 68:6519-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beatty, W. L., and P. J. Sansonetti. 1997. Role of lipopolysaccharide in signaling to subepithelial polymorphonuclear leukocytes. Infect. Immun. 65:4395-4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandenbug, K., G. Jurgens, M. Muller, S. Fukuoka, and M. H. J. Koch. 2001. Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin. Biol. Chem. 382:1215-1225. [DOI] [PubMed] [Google Scholar]
- 10.Brines, R. D., and J. H. Brock. 1983. The effect of trypsin and chymotrypsin on the in vitro antimicrobial and iron-binding properties of lactoferrin in human milk and bovine colostrum. Unusual resistance of human apolactoferrin to proteolytic digestion. Biochim. Biophys. Acta 759:229-235. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Y., M. R. Smith, K. Thirumalai, and A. Zychlinsky. 1996. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 15:3853-3860. [PMC free article] [PubMed] [Google Scholar]
- 12.Clemens, J. D., B. Stanton, F. Stoll, N. S. Shahid, H. Banu, and A. K. M Alauddin Chowdhury. 1986. Breast feeding as a determinant of severity in shigellosis. Am. J. Epidemiol. 123:710-720. [DOI] [PubMed] [Google Scholar]
- 13.Crouch, S. P., K. J. Slater, and J. Fletcher 1992. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood 80:235-240. [PubMed] [Google Scholar]
- 14.Elass-Rochard, E., A. Roseana, D. Legrand, M. Trif, V. Salmon, C. Motas, J. Montreuil, and G. Spik. 1995. Lactoferrin lipopolysaccharide interactions: involvement of the 28-34 loop region of human lactoferrin in the high affinity binding to E. coli O55B5 lipopolysaccharide. Biochem. J. 312:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elass-Rochard, E., D. Legrand, V. Salmon, A. Roseanu, M. Trif, P. S. Tobias, J. Mazurier, and G. Spik. 1998. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect. Immun. 66:486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellison, R. T., III, and T. J. Giehl. 1991. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Investig. 88:1080-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellison, R. T., T. J. Giehl, and F. M. LaForce. 1988. Damage of the outer membrane of enteric gram negative bacteria by lactoferrin and transferrin. Infect. Immun. 56:2774-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellison, R. T., F. M. LaForce, T. J. Giehl, D. S. Boose, and B. E. Dunn. 1990. Lactoferrin and transferring damage of the gram negative outer membrane is modulated by Ca2+ and Mg2+. J. Gen. Microbiol. 136:1437-1446. [DOI] [PubMed] [Google Scholar]
- 19.Erdei, J., A. Forsgren, and A. S. Naidu. 1994. Lactoferrin binds to porins OmpF and OmpC in Escherichia coli. Infect. Immun. 62:1236-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etheridge, M. E., A. T. M. Shamsul Hoque, and D. A. Sack. 1996. Pathologic study of a rabbit model for shigellosis. Lab. Anim. Sci. 46:61-66. [PubMed] [Google Scholar]
- 21.Gomez, H. F., I. Herrera-Insua, M. M. Siddiqui, V. A. Diaz-Gonzalez, E. Caceres, D. S. Newburg, and T. G. Cleary. 2001. Protective role of human lactoferrin against invasion of Shigella flexneri M90T. Adv. Exp. Med. Biol. 501:457-467. [DOI] [PubMed] [Google Scholar]
- 21a.Gomez, H. F., T. J. Ochoa, L. G. Carlin, and T. G. Cleary. Human lactoferrin impairs virulence of Shigella flexneri. J. Infect. Dis., in press. [DOI] [PubMed]
- 22.Guerrero, L., J. J. Calva, A. L. Morrow, F. R. Velazquez, F. Tuz-Dzib, Y. Vidal, H. Ortega, H. Arroyo, T. G. Cleary, and L. K. Pickering. 1994. Asymptomatic Shigella infections in a cohort of Mexican children younger than two years of age. Pediatr. Infect. Dis. J. 13:597-602. [DOI] [PubMed] [Google Scholar]
- 23.Guigiano, L. G., S. T. Ribeiro, M. H. Vainstein, and C. J. Ulhoa. 1995. Free secretory component and lactoferrin of human milk inhibit the adhesion of enterotoxigenic E. coli. J. Med. Microbiol. 42:3-9. [DOI] [PubMed] [Google Scholar]
- 24.Haversen, L. A., I. Engberg, L. Baltzer, G. Dolphin, L. A. Hanson, and I. Mattsby-Baltzer. 2000. Human lactoferrin and peptides derived from a surface exposed helical region reduce experimental Escherichia coli urinary tract infection in mice. Infect. Immun. 68:5816-5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayani, K. C., M. L. Guerrero, A. L. Morrow, H. F. Gomez, D. K. Winsor, G. M. Ruiz-Palacios, and T. G. Cleary. 1992. Concentration of milk secretory immunoglobulin A against Shigella virulence plasmid-associated antigens as a predictor of symptom status in Shigella-infected breast-fed infants. J. Pediatr. 121:852-856. [DOI] [PubMed] [Google Scholar]
- 26.High, N., J. Mounier, M. C. Prevost, and P. J. Sansonetti. 1992. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 11:1991-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawasaki, Y., S. Tazume, K. Shimizu, H. Matsuzawa, S. Dosako, H. Isoda, M. Tsukiji, R. Fujimura, Y. Muranaka, and H. Isihida. 2000. Inhibitory effects of bovine lactoferrin on the adherence of enterotoxigenic Escherichia coli to host cells. Biosci. Biotechnol. Biochem. 64:348-354. [DOI] [PubMed] [Google Scholar]
- 28.Lee, W. J., J. L. Farmer, M. Hilty, and Y. B. Kim. 1998. The protective effects of lactoferrin feeding against endotoxin lethal shock in germfree piglets. Infect. Immun. 66:1421-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, J. H. K., J. Gallar, and R. T. Loving. 1996. Endogenous circadian rhythm of basal pupil size in rabbits. Investig. Ophthalmol. Vis. Sci. 37:2345-2349. [PubMed] [Google Scholar]
- 30.Machnicki, M., M. Zimecki, and T. Zagulski. 1993. Lactoferrin regulates the release of tumour necrosis factor alpha and interleukin 6 in vivo. Int. J. Exp. Pathol. 74:433-439. [PMC free article] [PubMed] [Google Scholar]
- 31.Mattsby-Baltzer, I., A. Roseanu, C. Motas, J. Elverfors, I. Engberg, and L. A. Hanson. 1996. Lactoferrin or a fragment thereof inhibits the endotoxin-induced interleukin-6 response in human monocytic cells. Pediatr. Res. 40:257-262. [DOI] [PubMed] [Google Scholar]
- 32.Menard, R., M. C. Prevost, P. Gounon, P. Sansonetti, and C. Dehio. 1996. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc. Natl. Acad. Sci. USA 93:1254-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menard, R., P. J. Sansonetti, and and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naidu, S. S., U. Svensson, A. R. Kishore, and A. S. Naidu. 1993. Relationship between antibacterial activity and porin binding of lactoferrin in Escherichia coli and Salmonella typhimurium. Antimicrob. Agents Chemother. 37:240-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nascimento de Araujo, A., and L. G. Giugiano. 2000. Human milk fractions inhibit the adherence of diffusely adherent E. coli (DAEC) and enteroaggregative E. coli (EAEC) to HeLa cells. FEMS Microbiol. Lett. 184:91-94. [DOI] [PubMed] [Google Scholar]
- 36.Nascimento de Araujo, A., and L. G. Giugliano. 2001. Lactoferrin and free secretory component of human milk inhibit the adhesion of enteropathogenic E. coli to HeLa cells. BMC Microbiol. 1:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiter, B., J. H. Brock, and E. D. Steel. 1975. Inhibition of Escherichia coli by bovine colostrum and post-colostral milk. II. The bacteriostatic effect of lactoferrin on a serum susceptible and serum resistant strain of E. coli. Immunology 28:83-95. [PMC free article] [PubMed] [Google Scholar]
- 38.Sallmann, F. R., S. Descamps, F. Pattus, V. Salmon, N. Branza, G. Spik, and D. Legrand. 1999. Porins OmpC and PhoE of Escherichia coli as specific cell-surface targets of human lactoferrin. Binding characteristics and biological effects. J. Biol. Chem. 274:16107-16114. [DOI] [PubMed] [Google Scholar]
- 39.Samson, R. R., C. Mirtle, and D. B. McClelland. 1980. The effect of digestive enzymes on the binding and bacteriostatic properties of lactoferrin and vitamin B12 binder in human milk. Acta Paediatr. Scand. 69:517-523. [DOI] [PubMed] [Google Scholar]
- 40.Sansonetti, P. J., J. Arondel, J. R. Cantey, M. C. Prevost, and M. Huerre. 1996. Infection of rabbit Peyer's patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect. Immun. 64:2752-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sansonetti, P. J., A. Ryter, P. Clerc, A. T. Maurelli, and J. Mounier. 1986. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect. Immun. 51:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, P. D., L. E. Smythies, M. Mosteller-Barnum, D. A. Sibley, M. W. Russell, M. Merger, M. T. Sellers, J. M. Orenstein, T. Shimada, M. F. Graham, and H. Kubagawa. 2001. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J. Immunol. 167:2651-2656. [DOI] [PubMed] [Google Scholar]
- 43.Stoll, B. J., R. I. Glass, M. I.Huq, M. U. Khan, H. Banu, and J. Holt. 1982. Epidemiologic and clinical features of patients infected with Shigella who attended a diarrheal disease hospital in Bangladesh. J. Infect. Dis. 146:177-183. [DOI] [PubMed] [Google Scholar]
- 44.Stuart, J., S. Norrell, and J. P. Harrington. 1984. Kinetic effect of human lactoferrin on the growth of Escherichia coli 0111. Int. J. Biochem. 16:1043-1047. [DOI] [PubMed] [Google Scholar]
- 45.Thirumalai, K., K. S. Kim, and A. Zychlinsky. 1997. IpaB, a Shigella flexneri invasin, colocalizes with interleukin-1 beta-converting enzyme in the cytoplasm of macrophages. Infect. Immun. 65:787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tigyi, Z., A. R. Kishore, J. A. Maeland, A. Forsgren, and A. S. Naidu. 1992. Lactoferrin-binding proteins in Shigella flexneri. Infect. Immun. 60:2619-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward, P. P., C. S. Piddington, G. A. Cunningham, X. Zhou, R. D. Wyatt, and O. M. Conneely. 1995. A system for production of commercial quantities of human lactoferrin: a broad spectrum natural antibiotic. Bio/Technology 13:498-503. [DOI] [PubMed] [Google Scholar]
- 48.Wassef, J. S., D. F. Keren, and J. L. Mailloux. 1989. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect. Immun. 57:858-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watarai, M., T. Tobe, M. Yoshikawa, and C. Sasakawa. 1995. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 14:2461-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wenneras, C., P. Ave, M. Huerre, J. Arondel, R. J. Ulevitch, J. C. Mathison, and P. Sansonetti. 2000. Blockade of CD14 increases Shigella-mediated invasion and tissue destruction. J. Immunol. 164:3214-3221. [DOI] [PubMed] [Google Scholar]
- 51.Yamauchi, K., M. Tomita, T. J. Giehl, and R. T. Ellison III. 1993. Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect. Immun. 61:719-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zagulski, T., P. Lipinski, A. Zagulska, S. Broniek, and Z. Jarzabek. 1989. Lactoferrin can protect mice against a lethal dose of E. coli in experimental infection in vivo. Br. J. Exp. Pathol. 70:697-704. [PMC free article] [PubMed] [Google Scholar]
- 53.Zychlinsky, A., C. Fitting, J. M. Cavaillon, and P. J. Sansonetti. 1994. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J. Clin. Investig. 94:1328-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zychlinsky, A., B. Kenny, M. C. Prevost, I. B. Holland, and P. J. Sansonetti. 1993. The ipaB gene of Shigella flexneri and macrophage-programmed cell death. Infect. Agents Dis. 2:212-214. [PubMed] [Google Scholar]
- 55.Zychlinsky, A., B. Kenny, R. Menard, M. C. Prevost, I. B. Holland, and P. J. Sansonetti. 1994. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol. Microbiol. 11:619-627. [DOI] [PubMed] [Google Scholar]
- 56.Zychlinsky, A., K. Thirumalai, J. Arondel, J. R. Cantey, A. O. Aliprantis, and P. J. Sansonetti. 1996. In vivo apoptosis in Shigella flexneri infections. Infect. Immun. 64:5357-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]