Abstract
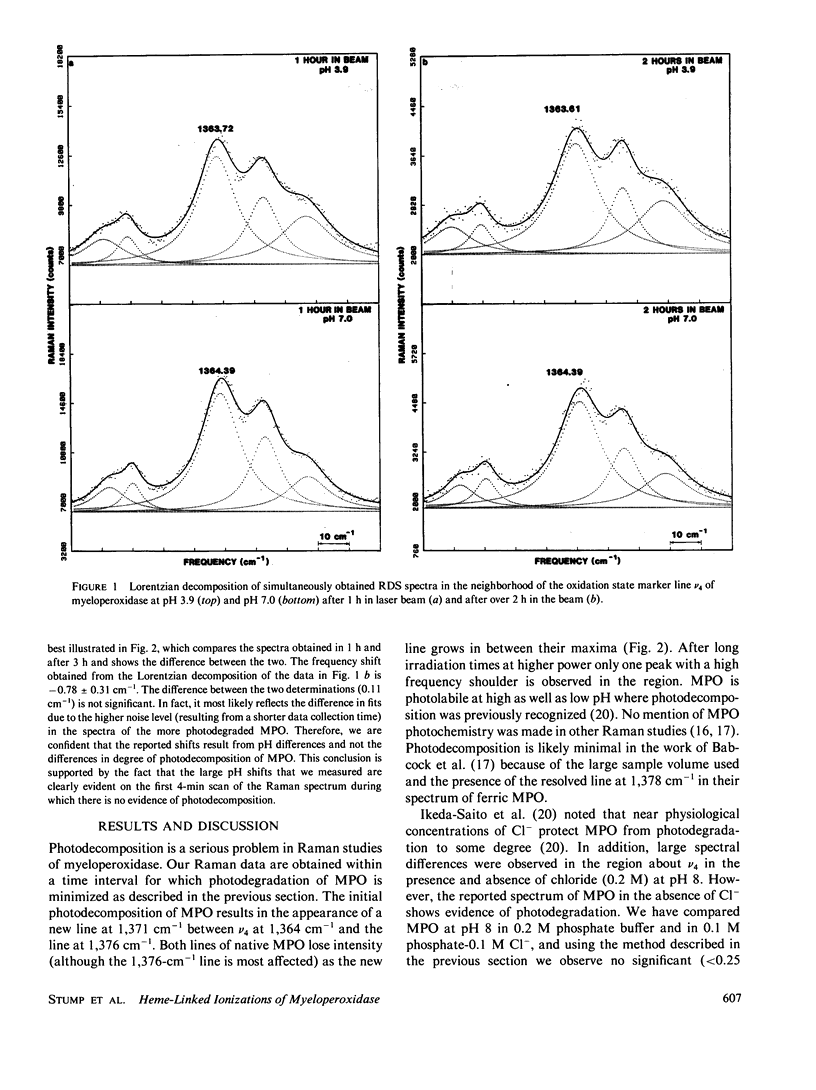
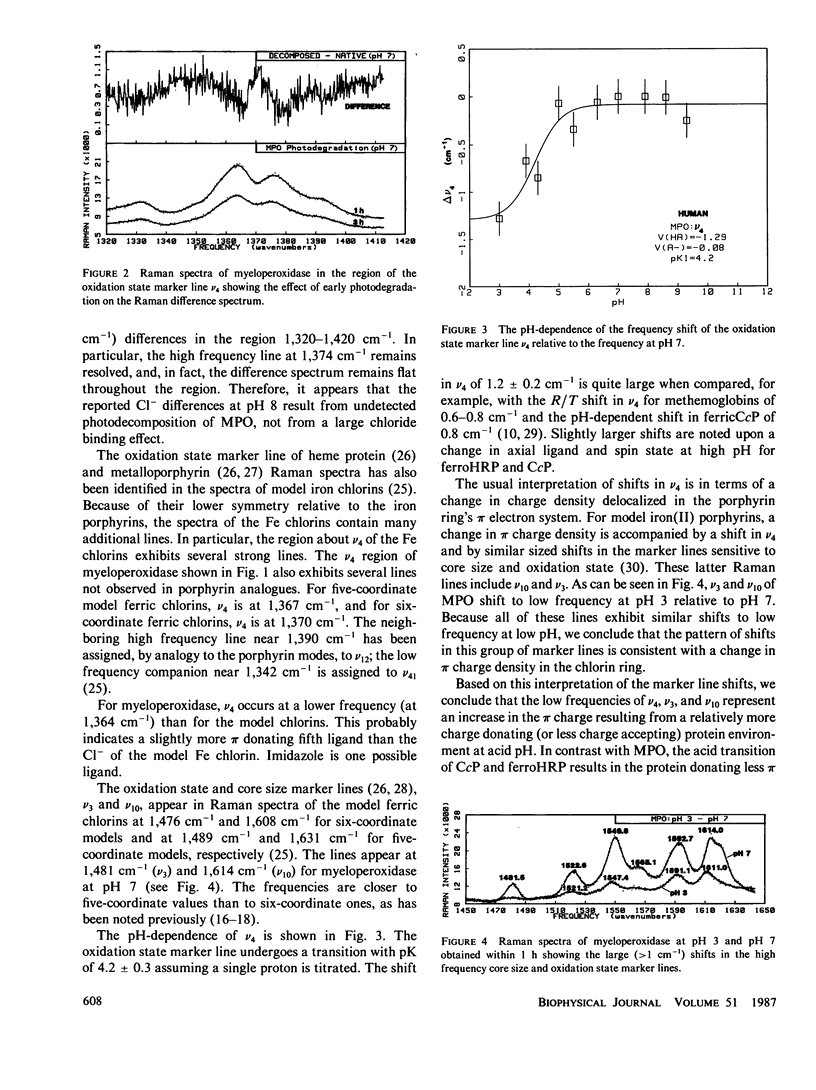
The pH-dependence of the oxidation state marker line v4 of human leucocyte myeloperoxidase is determined in the absence of chloride using Raman difference spectroscopy (RDS). A transition in the frequency of v4 with pK of 4.2 +/- 0.3 is found. The pK compares favorably with that previously determined by spectrophotometric titration and kinetic studies. The shift in v4 across the transition is -1.3 cm-1. The shift in v4 and other Raman marker lines indicates enhanced pi charge in the chlorin ring below the transition. The low frequencies of the oxidation state marker lines indicate that a structural change occurs near the chromophore, which results in the formation of a more pi-charge donating protein environment for the chlorin ring at low pH. The Raman results are discussed in terms of a proposed catalytic control mechanism based on charge stabilization of the energy of ring charge-depleted ferryl intermediates of the reaction with peroxide. The myeloperoxidase findings are compared with similar RDS results for ferrous horseradish peroxidase and ferric cytochrome c peroxidase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. C., Krinsky N. I. A kinetic analysis of the interaction of human myeloperoxidase with hydrogen peroxide, chloride ions, and protons. J Biol Chem. 1982 Nov 25;257(22):13240–13245. [PubMed] [Google Scholar]
- Babcock G. T., Ingle R. T., Oertling W. A., Davis J. C., Averill B. A., Hulse C. L., Stufkens D. J., Bolscher B. G., Wever R. Raman characterization of human leukocyte myeloperoxidase and bovine spleen green haemoprotein. Insight into chromophore structure and evidence that the chromophores of myeloperoxidase are equivalent. Biochim Biophys Acta. 1985 Mar 22;828(1):58–66. doi: 10.1016/0167-4838(85)90009-3. [DOI] [PubMed] [Google Scholar]
- Erman J. E. Kinetic and equilibrium studies of cyanide binding by cytochrome c peroxidase. Biochemistry. 1974 Jan 1;13(1):39–44. doi: 10.1021/bi00698a007. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Ikeda-Saito M., Argade P. V., Rousseau D. L. Resonance Raman evidence of chloride binding to the heme iron in myeloperoxidase. FEBS Lett. 1985 May 6;184(1):52–55. doi: 10.1016/0014-5793(85)80651-7. [DOI] [PubMed] [Google Scholar]
- Jensen M. S., Bainton D. F. Temporal changes in pH within the phagocytic vacuole of the polymorphonuclear neutrophilic leukocyte. J Cell Biol. 1973 Feb;56(2):379–388. doi: 10.1083/jcb.56.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Yamazaki I., Kitagawa T. Unusual low-frequency resonance Raman spectra of heme observed for hog intestinal peroxidase and its derivatives. Biochemistry. 1981 Aug 4;20(16):4632–4638. doi: 10.1021/bi00519a018. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Hashimoto S., Teraoka J., Nakamura S., Yajima H., Hosoya T. Distinct heme-substrate interactions of lactoperoxidase probed by resonance Raman spectroscopy: difference between animal and plant peroxidases. Biochemistry. 1983 Jun 7;22(12):2788–2792. doi: 10.1021/bi00281a003. [DOI] [PubMed] [Google Scholar]
- Lent B., Conroy C. W., Erman J. E. The effect of ionic strength on the kinetics of fluoride binding to cytochrome c peroxidase. Arch Biochem Biophys. 1976 Nov;177(1):56–61. doi: 10.1016/0003-9861(76)90415-x. [DOI] [PubMed] [Google Scholar]
- Loo S., Erman J. E. A kinetic study of the reaction between cytochrome c peroxidase and hydrogen peroxide. Dependence on pH and ionic strength. Biochemistry. 1975 Jul 29;14(15):3467–3470. doi: 10.1021/bi00686a027. [DOI] [PubMed] [Google Scholar]
- Rousseau D. L., Shelnutt J. A., Henry E. R., Simon S. R. Raman difference spectroscopy of tertiary and quaternary structure changes in methaemoglobins. Nature. 1980 May 1;285(5759):49–51. doi: 10.1038/285049a0. [DOI] [PubMed] [Google Scholar]
- Schultz J., Corlin R., Oddi F., Kaminker K., Jones W. Myeloperoxidase of the leucocyte of normal human blood. 3. Isolation of the peroxidase granule. Arch Biochem Biophys. 1965 Jul;111(1):73–79. doi: 10.1016/0003-9861(65)90324-3. [DOI] [PubMed] [Google Scholar]
- Shelnutt J. A., Alden R. G., Ondrias M. R. Heme-linked ionizations in horseradish peroxidase detected by Raman difference spectroscopy. J Biol Chem. 1986 Feb 5;261(4):1720–1723. [PubMed] [Google Scholar]
- Shelnutt J. A., Rousseau D. L., Dethmers J. K., Margoliashi E. Protein influence on the heme in cytochrome c: evidence from Raman difference spectroscopy. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3865–3869. doi: 10.1073/pnas.76.8.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelnutt J. A., Satterlee J. D., Erman J. E. Raman difference spectroscopy of heme-linked ionizations in cytochrome c peroxidase. J Biol Chem. 1983 Feb 25;258(4):2168–2173. [PubMed] [Google Scholar]
- Sibbett S. S., Hurst J. K. Structural analysis of myeloperoxidase by resonance Raman spectroscopy. Biochemistry. 1984 Jun 19;23(13):3007–3013. doi: 10.1021/bi00308a025. [DOI] [PubMed] [Google Scholar]
- Spiro T. G., Burke J. M. Protein control of porphyrin conformation. Comparison of resonance Raman spectra of heme proteins with mesoporphyrin IX analogues. J Am Chem Soc. 1976 Sep 1;98(18):5482–5489. doi: 10.1021/ja00434a013. [DOI] [PubMed] [Google Scholar]
- Stelmaszyńska T., Zgliczyński J. M. Myeloperoxidase of human neutrophilic granulocytes as chlorinating enzyme. Eur J Biochem. 1974 Jun 1;45(1):305–312. doi: 10.1111/j.1432-1033.1974.tb03555.x. [DOI] [PubMed] [Google Scholar]
- Teraoka J., Kitagawa T. Structural implication of the heme-linked ionization of horseradish peroxidase probed by the Fe-histidine stretching Raman line. J Biol Chem. 1981 Apr 25;256(8):3969–3977. [PubMed] [Google Scholar]
- Yamada H., Makino R., Yamazaki I. Effects of 2,4-substituents of deuteropheme upon redox potentials of horseradish peroxidases. Arch Biochem Biophys. 1975 Jul;169(1):344–353. doi: 10.1016/0003-9861(75)90350-1. [DOI] [PubMed] [Google Scholar]
- Yamada H., Yamazaki I. Proton balance in conversions between five oxidation-reduction states of horseradish peroxidase. Arch Biochem Biophys. 1974 Dec;165(2):728–738. doi: 10.1016/0003-9861(74)90301-4. [DOI] [PubMed] [Google Scholar]
- Zgliczynski J. M., Selvaraj R. J., Paul B. B., Stelmaszynska T., Poskitt P. K., Sbarra A. J. Chlorination by the myeloperoxidase-H2O2-Cl- antimicrobial system at acid and neutral pH. Proc Soc Exp Biol Med. 1977 Mar;154(3):418–422. doi: 10.3181/00379727-154-39684. [DOI] [PubMed] [Google Scholar]


