Abstract
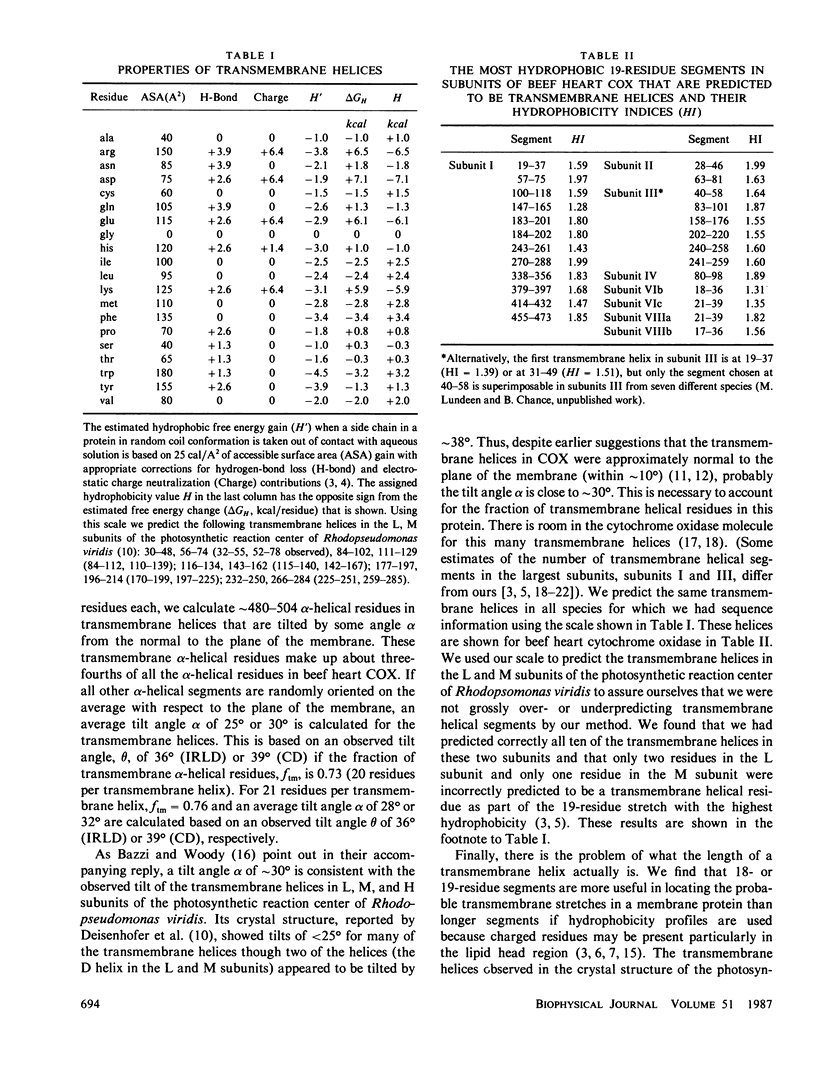
The locations of the transmembrane helices in the 12 subunits of beef heart cytochrome oxidase were predicted with a modified form of the von Heijne-Blomberg hydrophobicity scale. Based on ∼20 residues per transmembrane helix, about 480 of the estimated 660 helical residues (36.8% of 1,793 total residues) are expected to be in transmembrane helices that have their axes tilted by a small angle α from the normal to the plane of the membrane. This angle is calculated to be ∼30°, based on the observed overall tilt angle θ of 39° obtained from circular dichroism (CD) measurements on multilamellar films, or about 25°, based on the observed tilt angle θ of 36° obtained from the infrared linear dichroism of films. For 21 residues per transmembrane helix, the calculated values of α become 32° and 28°, respectively, depending upon the value of θ used. Thus, a transmembrane helical tilt angle of ∼30° accounts for the predicted transmembrane stretches in cytochrome oxidase if 20-21 residues are sufficient to span the membrane. Additional helical residues in the lipid head region may deviate by a larger angle from the normal to the plane of the membrane in cytochrome oxidase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazzi M. D., Woody R. W. Oriented secondary structure in integral membrane proteins. I. Circular dichroism and infrared spectroscopy of cytochrome oxidase in multilamellar films. Biophys J. 1985 Dec;48(6):957–966. doi: 10.1016/S0006-3495(85)83859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi M. D., Woody R. W. Oriented secondary structure in integral membrane proteins. I. Circular dichroism and infrared spectroscopy of cytochrome oxidase in multilamellar films. Biophys J. 1985 Dec;48(6):957–966. doi: 10.1016/S0006-3495(85)83859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson R., Montecucco C. Helical packing in the hydrophobic sector of cytochrome c oxidase. J Inorg Biochem. 1985 Mar-Apr;23(3-4):177–182. doi: 10.1016/0162-0134(85)85023-6. [DOI] [PubMed] [Google Scholar]
- Bisson R., Steffens G. C., Buse G. Localization of lipid binding domain(s) on subunit II of beef heart cytochrome c oxidase. J Biol Chem. 1982 Jun 25;257(12):6716–6720. [PubMed] [Google Scholar]
- Blasie J. K., Erecińska M., Samuels S., Leigh J. S. The structure of a cytochrome oxidase-lipid model membrane. Biochim Biophys Acta. 1978 Jan 11;501(1):33–52. doi: 10.1016/0005-2728(78)90093-2. [DOI] [PubMed] [Google Scholar]
- Buse G., Meinecke L., Bruch B. The protein formula of beef heart cytochrome c oxidase. J Inorg Biochem. 1985 Mar-Apr;23(3-4):149–153. doi: 10.1016/0162-0134(85)85019-4. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Malatesta F., Darley-Usmar V. M. Structure of cytochrome c oxidase. Biochim Biophys Acta. 1983 Jul 15;726(2):135–148. doi: 10.1016/0304-4173(83)90003-4. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Malatesta F., Darley-Usmar V. M. Structure of cytochrome c oxidase. Biochim Biophys Acta. 1983 Jul 15;726(2):135–148. doi: 10.1016/0304-4173(83)90003-4. [DOI] [PubMed] [Google Scholar]
- Deatherage J. F., Henderson R., Capaldi R. A. Relationship between membrane and cytoplasmic domains in cytochrome c oxidase by electron microscopy in media of different density. J Mol Biol. 1982 Jul 5;158(3):501–514. doi: 10.1016/0022-2836(82)90211-x. [DOI] [PubMed] [Google Scholar]
- Erdweg M., Buse G. Studies on cytochrome c oxidase, XI. The amino-acid sequence of bovine heart polypeptide VIc. Biol Chem Hoppe Seyler. 1985 Mar;366(3):257–263. doi: 10.1515/bchm3.1985.366.1.257. [DOI] [PubMed] [Google Scholar]
- Frey T. G., Kuhn L. A., Leigh J. S., Jr, Costello M. J., Chan S. H. Cytochrome oxidase: structural insights from electron microscopy and from secondary structure prediction. J Inorg Biochem. 1985 Mar-Apr;23(3-4):155–162. doi: 10.1016/0162-0134(85)85020-0. [DOI] [PubMed] [Google Scholar]
- Henderson R. The purple membrane from Halobacterium halobium. Annu Rev Biophys Bioeng. 1977;6:87–109. doi: 10.1146/annurev.bb.06.060177.000511. [DOI] [PubMed] [Google Scholar]
- Keniry M. A., Gutowsky H. S., Oldfield E. Surface dynamics of the integral membrane protein bacteriorhodopsin. 1984 Jan 26-Feb 1Nature. 307(5949):383–386. doi: 10.1038/307383a0. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Malatesta F., Darley-Usmar V., de Jong C., Prochaska L. J., Bisson R., Capaldi R. A., Steffens G. C., Buse G. Arrangement of subunit IV in beef heart cytochrome c oxidase probed by chemical labeling and protease digestion experiments. Biochemistry. 1983 Sep 13;22(19):4405–4411. doi: 10.1021/bi00288a010. [DOI] [PubMed] [Google Scholar]
- Michel H., Weyer K. A., Gruenberg H., Dunger I., Oesterhelt D., Lottspeich F. The 'light' and 'medium' subunits of the photosynthetic reaction centre from Rhodopseudomonas viridis: isolation of the genes, nucleotide and amino acid sequence. EMBO J. 1986 Jun;5(6):1149–1158. doi: 10.1002/j.1460-2075.1986.tb04340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabedryk E., Breton J. Orientation of intrinsic proteins in photosynthetic membranes. Polarized infrared spectroscopy of chloroplasts and chromatophores. Biochim Biophys Acta. 1981 May 13;635(3):515–524. doi: 10.1016/0005-2728(81)90110-9. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A. Polarized infrared spectroscopy of oriented purple membrane. Biophys J. 1979 Mar;25(3):473–487. doi: 10.1016/S0006-3495(79)85317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welinder K. G., Mikkelsen L. The oxygen binding site of cytochrome oxidase. Structural predictions on subunit I from amino acid sequences. FEBS Lett. 1983 Jul 4;157(2):233–239. doi: 10.1016/0014-5793(83)80553-5. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Blomberg C. Trans-membrane translocation of proteins. The direct transfer model. Eur J Biochem. 1979 Jun;97(1):175–181. doi: 10.1111/j.1432-1033.1979.tb13100.x. [DOI] [PubMed] [Google Scholar]


