Although Canadian guidelines for the diagnosis and management of asthma have been published over the last 15 years,1,2,3,4 there has been little focus on issues relevant to asthma in the young child or on prevention strategies for asthma. Since the last update in 2001,4 important issues and new studies focusing on asthma in early life have highlighted the need to incorporate the new information into the asthma guidelines. Reports pertaining to a number of issues published between 2000 and June 2003 were reviewed initially by small expert resource groups. The results of these reviews were discussed by stakeholders during a 2-day consensus meeting, 27–28 June 2003. A working group with a pediatric focus met under the auspices of the Canadian Network For Asthma Care and an adult asthma group met under the auspices of the Canadian Thoracic Society. On the first day, these groups met separately to discuss specific issues related to pediatric and adult asthma and, on the second day, met jointly to discuss dissemination and implementation of the asthma guidelines. Data published up to December 2004 pertaining to each of the issues considered by the consensus working group were reviewed by the individual expert resource groups, who concurred that these were insufficient to modify any of the recommendations that follow.
This summary reports the recommendations for prevention, assessment and management of asthma in children and adults. A level of evidence is assigned to each recommendation based on the strength of the supporting data5 (Table 1). Background documents supporting recommendations for children follow in a separate supplement. Background documents for adults are published in the Canadian Respiratory Journal.6
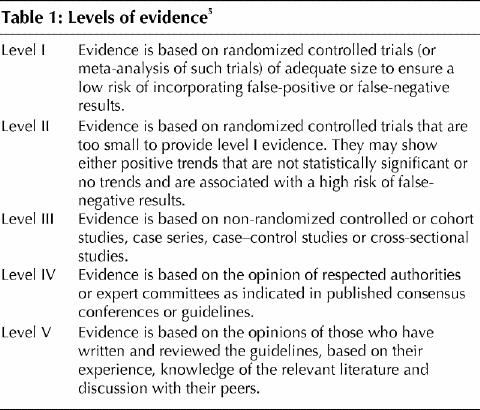
Table 1
Definition of asthma
The definition of asthma remains descriptive and has not changed since the 1999 Canadian asthma consensus guidelines.3 Asthma is characterized by paroxysmal or persistent symptoms, such as dyspnea, chest tightness, wheezing, sputum production and cough associated with variable airflow limitation and airway hyperresponsiveness to endogenous or exogenous stimuli. Inflammation and its resultant effects on airway structure are considered the main mechanisms leading to the development and persistence of asthma.
General management of asthma
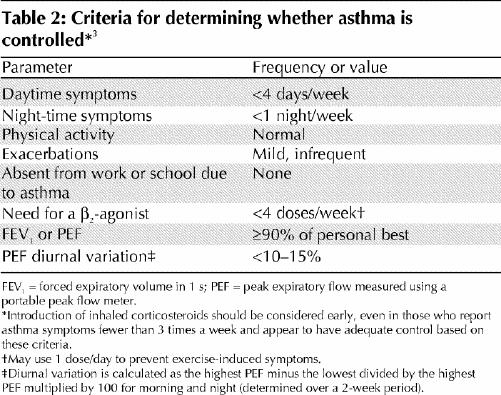
Optimal management of asthma requires adequate evaluation of the patient and his or her environment. Asthma control should be assessed using specific criteria (Table 2). Asthma severity is more difficult to assess and may only be determined after asthma control is achieved. Asthma control should be assessed at each visit.
Table 2
If control is inadequate, the reason or reasons should be identified and maintenance therapy should be modified (Fig. 1). Any new treatment should be considered a therapeutic trial and its effectiveness should be re-evaluated after 4–6 weeks.
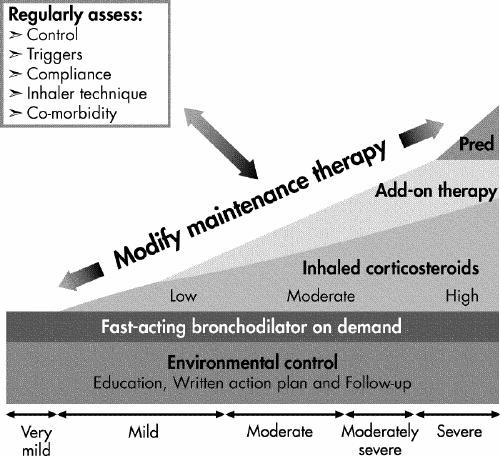
Inhaled corticosteroids (ICSs) should be introduced as initial maintenance treatment even when the patient reports symptoms fewer than 3 times a week. Although less effective than low-dose ICSs, leukotriene receptor antagonists (LTRAs) are an alternative for patients who cannot or will not use ICSs. If control is inadequate on low-dose ICSs, identify the reasons for poor control and, if indicated, consider additional therapy with long-acting β2-agonists or LTRAs. Severe asthma may require additional treatment with systemic steroids. Asthma control and maintenance therapy must be assessed regularly.
If good control has been sustained, consideration should be given to gradually reducing maintenance therapy, with regular reassessments to ensure that control remains adequate. This will allow determination of the minimum therapy needed to maintain acceptable asthma control.
Asthma education is an essential component of asthma care. Poor asthma control is not usually due to a lack of efficacy of the medication, but is more often related to suboptimal use of medication or aggravating factors, comorbidities, poor inhaler technique, poor environmental control or a lack of continuity of care. Suboptimal use of asthma medication may be the result of inappropriate physician recommendation, poor adherence or both, perhaps as a result of undue fear of adverse effects of therapy. In the face of poor asthma control, it is crucial to identify and address the cause (Table 3).
Table 3

Diagnosis of asthma
Recommendations regarding the diagnosis or assessment of asthma severity in adults and older children have not changed from previous publications.3 However, the diagnosis of asthma in the preschool child was a major focus of the current discussions.
Recommendations
Physicians must obtain an appropriate patient and family history to assist them in recognizing the heterogeneity of wheezing phenotypes in preschool-aged children (level III).
In children who are unresponsive to asthma therapy, physicians must exclude other pathology that might suggest an alternative diagnosis (level IV).
The presence of atopy should be determined because it is a predictor of persistent asthma (level III).
Diagnostic tools
In children <3 years of age, neither lung function testing nor assessment of airway inflammation is clinically helpful nor commonly available for the diagnosis of asthma.4,7,8,9 Asthma diagnosis in children <6 years of age is dependent on history and physical examination. Table 4 provides some criteria to help identify a child suffering from asthma; the greater the number of criteria met, the greater the likelihood of asthma.
Table 4

Evolution of preschool wheezing
Preschool wheezing can be classified as transient early onset wheezing (before age 3 years), which is often outgrown; persistent early onset wheezing (before age 3 years), which persists in school age; and late-onset wheezing (after age 3 years), which is less likely to resolve. Among preschool children with wheezing, 50%–60% outgrow the problem.10,11
Role of atopy
Recurrent wheezing in non-atopic preschool children is likely to resolve in childhood, but atopy is a predictor of persistent asthma.12,13,14 A clinical index may help predict which wheezing children are likely to have persistent asthma15 (Table 5). Physicians must obtain a personal and family history of atopy and look specifically for the presence of atopic dermatitis during physical examination. The presence of atopy can be established by skin-prick testing16 or measurement of specific IgE antibodies17,18 and is suggested by elevated peripheral total IgE and blood eosinophils.18,19,20
Table 5

Prevention strategies
Recommendations
Primary prevention
With conflicting data on early life exposure to pets, no general recommendation can be made with regard to avoiding pets for primary prevention of allergy and asthma (level III). However, families with biparental atopy should avoid having cats or dogs in the home (level II).
There are conflicting and insufficient data for physicians to recommend for or against breastfeeding specifically for the prevention of asthma (level III). Due to its numerous other benefits, breastfeeding should be recommended.
Secondary prevention
3. Health care professionals should continue to recommend the avoidance of tobacco smoke in the environment (level IV).
4. For patients sensitized to house dust mites, physicians should encourage appropriate environmental control (level V).
5. In infants and children who are atopic, but do not have asthma, data are insufficient for physicians to recommend other specific preventive strategies (level II).
Tertiary prevention
6. Allergens to which a person is sensitized should be identified (level I), and a systematic program to eliminate, or at least to substantially reduce, allergen exposure in sensitized people should be undertaken (level II).
Primary prevention
Primary prevention of asthma is defined as intervention before the development of asthma or any predisposing disease such as atopic dermatitis, food allergy or allergic rhinitis. We focused on 2 specific areas of primary prevention: exposure to pets in early life and breastfeeding. Recommendations related to avoiding exposure to environmental tobacco smoke remain unchanged.
Sensitization to allergens is one of the strongest determinants of subsequent development of asthma. Several recent studies have suggested the possibility that a cat or dog in the home early in life might decrease the risk of developing allergy or asthma.21,22,23,24,25,26,27 Currently available data do not provide conclusive guidance relating to exposure to pets in early life. There is evidence that children with biparental atopy and those whose mothers have asthma should avoid exposure to pets in early life.26
There is clear evidence that breastfeeding protects against early life wheezing syndromes.28,29 However, recent studies suggest that breastfeeding may increase the risk of persistent asthma.30,31 Reasons for this are speculative, but may relate to a lower incidence of infectious diseases among breastfed children (the hygiene hypothesis) or to a higher rate of breastfeeding in atopic families (confounding by indication). However, other benefits of breastfeeding are sufficiently clear to recommend exclusive breastfeeding of infants for the first 4 months of life or more.
Secondary prevention
Secondary prevention is defined as intervention(s) for infants and children who are at high risk for the development of asthma but who have not yet developed asthma symptoms or signs.32 These patients usually have allergic conditions and a family history of allergic disease.32 There is currently insufficient evidence regarding pharmacologic treatment, control of environmental factors or allergen-specific immunotherapy to allow firm recommendations to be made. Health care personnel should continue to recommend smoke avoidance measures in all children and reduction of dust mites in the environment of sensitized people.
Tertiary prevention
Tertiary prevention implies identifying allergens to which a person is sensitized and undertaking a systematic program to eliminate, or at least substantially reduce, allergen exposure in sensitized people. This strategy is still endorsed.
Pharmacotherapy
Recommendations
First-line maintenance therapy
Physicians should recommend inhaled corticosteroids (ICSs) as the best option for anti-inflammatory monotherapy for childhood asthma (level I).
There is insufficient evidence to recommend leukotriene receptor antagonists (LTRAs) as first-line monotherapy for childhood asthma (level I). For children who cannot or will not use ICSs, LTRAs represent an alternative (level II).
Treatment of intermittent asthma with ICSs
3. There are insufficient data for physicians to recommend short courses of high-dose ICSs in children with mild, intermittent asthma symptoms, and the safety of this approach has not been established (level II).
4. Physicians must carefully monitor children with intermittent symptoms to ensure that they do not develop chronic symptoms requiring maintenance therapy (level IV).
5. Physicians should recommend that children with frequent symptoms, severe asthma exacerbations or both receive regular, not intermittent, treatment with ICSs (level IV).
Add-on therapies
6. Long-acting β2-agonists are not recommended as maintenance monotherapy in asthma (level I).
7. After reassessment of compliance, control of environment and diagnosis, if asthma is not optimally controlled with moderate doses of ICSs, therapy should be modified by the addition of a long-acting β2-agonist (level I). Alternatively, addition of an LTRA or increasing to a moderate dose of ICS may be considered (level I).
Relievers
Short-acting β2-agonists have been used for symptom relief for many years.33 Recently, a long- but also fast-acting agent, formoterol, has been approved for symptom relief.34 Fast-acting bronchodilators may be used to relieve acute intermittent asthma symptoms. They should be used only on demand at the minimum dose and frequency required. Need for a reliever more than 3 times a week (aside from a pre-exercise dose) suggests suboptimal asthma control and indicates the need to re-assess treatment. Inhaled ipratropium bromide is less effective, but in the emergency department, ipratropium bromide combined with fast-acting β2-agonists is effective for treatment of severe acute asthma in children and adults.35,36
First-line maintenance therapy
Early ICS treatment
The role of ICSs in the early treatment of mild-to-moderate asthma has been extensively evaluated. In a systematic review,37 treatment with beclomethasone significantly improved forced expiratory volume in 1 s (FEV1) and morning peak expiratory flow (PEF) and reduced the use of β-agonists and exacerbations compared with placebo. In a recent large prospective study of pediatric and adult patients with mild asthma, early use of moderate-dose inhaled budesonide was associated with better control of symptoms, improved FEV1 and, importantly, a marked reduction in asthma exacerbations compared with placebo.38
ICS therapy may be associated with mild reductions in linear growth in children,39 which appears to occur primarily during the first year of therapy. Prospective studies show that children treated with moderate doses of ICS for long periods of time attain their predicted adult height.40 There is no evidence to support initial treatment using combination therapy (ICS and long-acting β-agonist) in patients not previously given a trial of an ICS alone.41
An alternative to ICS is use of a leukotriene receptor antagonist (LTRA). Three well-designed trials, 1 in preschool-aged and 2 in school-aged children demonstrated the superiority of LTRA over placebo in persistent asthma.42,43,44 Montelukast was associated with fewer days of asthma symptoms and β2-agonist use, less use of rescue oral steroids and, in older children, greater improvement in lung function.
A Cochrane review of moderate-dose ICS compared with LTRA monotherapy in school-aged children with mild-to-moderate airway obstruction reported less use of β2-agonist in the ICS group but no significant difference in symptoms, spirometry or the risk of an asthma exacerbation requiring systemic steroids.45 There are currently too few trials to draw any firm conclusions. A recent systematic review comparing ICS (400 mg of beclomethasone or equivalent) to LTRAs in mild-to-moderate asthma identified 13 trials (all adult trials with 1 exception). This review found that adults treated with LTRAs were more likely to suffer an asthma exacerbation requiring a course of oral prednisone.46 Thus, ICSs remain the preferred initial treatment for asthma in children and adults.
The effectiveness of intermittent treatment
Intermittent asthma symptoms are a common pattern of asthma in infants and children, with exacerbations usually triggered by viral, upper respiratory tract infections. This form of asthma is less likely associated with atopy and may have a different natural history. Treatment is problematic, as optimal therapy has not been clearly determined. Because such children are asymptomatic between exacerbations, intermittent treatment with ICS is attractive to both physicians and families, and this management strategy is prevalent in Canada even though evidence to support the practice is scant.
Studies performed in preschool-aged children using high-dose intermittent therapy (beclomethasone 2250 μg/day, or budesonide 1600–3200 μg/day), for 5–10 days showed small reductions in asthma symptom scores and a trend toward less use of oral steroids. However, duration of symptoms, emergency visits and admissions to hospital did not appear to be affected by intermittent high-dose ICSs.47,48,49,50 Few studies evaluated the safety of intermittent high-dose ICS treatment.51
Therapy in addition to ICSs
In patients whose asthma is not adequately controlled on ICSs, available therapeutic options include add-on therapy with a long-acting β2-agonist, LTRA or theophylline or increasing the dose of ICS.
Long-acting β2-agonists
Long-acting β2-agonists are safe and effective medications for improving asthma control in older children and adults with asthma not optimally controlled despite regular maintenance therapy with ICSs,41,52,53 but they should not be used as monotherapy.54
Leukotriene receptor antagonists
In children and adults treated with moderate dose ICSs, there is some evidence suggesting that adding LTRAs is associated with improvements similar to doubling the ICS dose, but there is not yet sufficient evidence of equivalence between the 2 therapeutic strategies.55
Theophylline
In the few studies available evaluating add-on therapy in patients on ICS, theophylline was less effective than long-acting β2-agonists or LTRAs for improving asthma control.56
Comparison of long-acting β2-agonists and LTRAs as add-on therapy to ICSs
In adults, the addition of long-acting β2-agonists to 400 μg of chlorofluorocarbon-propelled beclomethasone or equivalent is more effective than LTRAs for improving lung function, reducing symptoms and use of rescue β2-agonists. However, both treatments had similar rates of asthma exacerbations and adverse events were similar in both groups.57,58,59 No similar data are yet available for children.
Inhalation devices
Only inhalation devices for childhood asthma were reviewed.
Recommendations
At each contact, health care professionals should work with patients and their families on inhaler technique (level I).
When prescribing a pressurized metered-dose inhaler (pMDI) for maintenance or acute asthma, physicians should recommend use of a valved spacer, with mouthpiece when possible, for all children (level II).
Although physicians should allow children choice of inhaler device, breath-actuated devices such as dry- powder inhalers offer a simpler option for maintenance treatment in children over 5 years of age (level IV).
Children tend to “auto-scale” their inhaled medication dose and the same dose of maintenance medication can be used at all ages for all medications (level IV).
Physicians, educators and families should be aware that jet nebulizers are rarely indicated for the treatment of chronic or acute asthma (level I). Delivery of medicinal aerosols depends on adequate inhalation technique. After repeated instruction and demonstration, more than 90% of children are able to achieve correct inhalation technique.60,61 Better knowledge of asthma, increased satisfaction with education and diminished asthma instability and attacks are associated with improved inhalation technique.62,63
One of the most difficult inhalation techniques to master is the use of a pressurized metered-dose inhaler (pMDI).64,65 Use of a spacer with pMDIs is strongly recommended for children. The pMDI with spacer can be used in place of the wet nebulizer in children of all ages in both acute and chronic care settings.66,67,68,69,70,71 Use of a mouthpiece, rather than a mask, (generally at 4 or 5 years old) maximizes lung deposition.72
Children can generally use dry-powder inhalers (DPIs), such as Turbuhaler and Diskus by the age of 5–6 years.73,74,75 Adults prefer breath-actuated DPIs over pMDIs and perform better with them.64 Using more than one inhalation device, may worsen technique with each device.76
In young children, deposition of medication in the lungs is about a tenth of the dose that would be delivered in adults. Thus, the same dose of maintenance medication can be used at all ages, because it will be “auto-scaled” down in children.77,78
Immunotherapy
The literature on immunotherapy was reviewed only for childhood asthma and the current recommendations are directed toward children.
Recommendations
Physicians should consider injection immunotherapy using appropriate allergens for the treatment of allergic asthma only when the allergic component is well documented (level I).
Physicians should not recommend the use of injection immunotherapy in place of avoidance of environmental allergens (level III).
Physicians may consider injection immunotherapy in addition to appropriate environmental control and pharmacotherapy when asthma control remains inadequate (level IV).
Immunotherapy is not recommended when asthma is unstable (level III).
Immune modulation is the only currently available therapy aimed at modifying the underlying disease process in asthma. Allergen immunotherapy is defined by the World Health Organization as therapeutic vaccine(s) for allergic diseases.79 Although debate about the value of immunotherapy continues, meta-analysis and review of immunotherapy support the potential value in childhood.80 Early immunotherapy may prevent development of asthma in children sensitized to house dust mite allergen.81 Allergen immunotherapy should be combined with allergen avoidance, pharmacotherapy and patient education. Furthermore, appropriate immunotherapy requires the use of single, well-defined allergens reaching a sufficient final dose to ensure effectiveness. As commonly undertaken, the value of immunotherapy using multiple allergens remains suspect.
Education and follow-up
Recommendations
Education is an essential component of asthma therapy and should be offered to all patients; educational interventions may be of particular benefit in patients with high asthma-related morbidity or severe asthma and at the time of emergency department visits and admissions to hospital (level I). Education programs should be evaluated (level III).
All patients should monitor their asthma using symptoms or peak expiratory flow (PEF) measurement (level I) and have written action plans for self-management that include medication adjustment in response to severity or frequency of symptoms, the need for symptom relief medication or a change in PEF (level I).
Asthma control criteria should be assessed at each visit (level IV). Measurement of pulmonary function, preferably by spirometry, should be done regularly (level III) in adults and children 6 years of age and older.
Socioeconomic and cultural factors should be taken into account in designing asthma education programs (level II).
Asthma education is an important part of asthma management and should aim primarily at changing patient behaviour, rather than simply improving knowledge.82 Patients with marked asthma-related morbidity and frequent acute care use should be targeted for asthma education. In this population, structured education with a written self-management plan, regular medical reassessment and review of key concepts reduces the number of emergency department visits.82,83,84
Recent studies, including a meta-analyses in children and adults, have confirmed that various methods of asthma education can improve symptoms, emotional state, communication with family members, school and physicians, school absenteeism, activity restriction, self-management skills, morbidity, lung function, quality of life, exacerbation rates and need for oral corticosteroids.85,86,87,88,89,90,91,92,93,94,95,96,97 Beneficial effects have been observed in a study involving adolescents, using education provided by peers.93 Long-term outcome may be improved further by reinforcement visits.91 Internet-based education may also improve adherence to the treatment plan in children. Education improves adherence to some environmental control measures, such as dust mite reduction measures, but is less helpful for animal avoidance in sensitized subjects.90
Conclusion
In Canadian children and adults with asthma, poor control remains prevalent, resulting in preventable morbidity, acute care visits, admission to hospital and even, fortunately rarely, mortality.98 In many cases, poor asthma outcomes can be avoided by ensuring that ICSs are started early and used as regular, long-term maintenance therapy, with special care taken to ensure patient compliance. Other crucial elements to achieving and maintaining good asthma control (Table 2) are environmental control measures, asthma education, treatment of comorbidity and appropriate use of add-on therapies.
It is hoped that these guidelines will improve asthma control in the many Canadians coping with this far too common disease.
Footnotes
*Members of the Pediatric Asthma Working Group of the Canadian Network For Asthma Care:
Mary L. Allen, MA, Allergy/Asthma Information Association, Montréal, Que.
Pierre Beaudry, MD, Canada Pediatric Society, Ottawa, Ont.
Allan Becker, MD, University of Manitoba, Winnipeg, Man.
Melva Bellafontaine, The Asthma Society of Canada, Toronto, Ont.
Denis Bérubé, MD, University of Montréal, Montréal, Que.
Andrew Cave, MD, University of Alberta, Edmonton, Alta.
Zave Chad, MD, University of Ottawa, Ottawa, Ont.
Myrna Dolovich, PEng, McMaster University, Hamilton, Ont.
Francine M. Ducharme, MD, McGill University, Montréal, Que.
Tony D'Urzo, MD, University of Toronto, Toronto, Ont.
Pierre Ernst, MD, MSc, McGill University, Montréal, Que.
Alexander Ferguson, MB, ChB, University of British Columbia, Vancouver, B.C.
Cathy Gillespie, RN, MN, CAE, Health Sciences Centre, Winnipeg, Man.
Mark Greenwald, MD, Asthma Society of Canada, Toronto, Ont.
Donna Hogg, RN, CAE, Canadian Network For Asthma Care, Edmonton, Alta.
Andrea Hudson, PhD, Canadian Pharmacists Association, Toronto, Ont.
Alan Kaplan, MD, Canadian Family Physician's Asthma Group, Richmond Hill, Ont.
Sandeep Kapur, MD, Dalhousie University, Halifax, N.S.
Cheryle Kelm, BPT, MSc, University of Calgary, Calgary, Alta.
Thomas Kovesi, MD, University of Ottawa, Ottawa, Ont.
Brian Lyttle, MD, University of Western Ontario, London, Ont.
Bruce Mazer, MD, McGill University, Montréal, Que.
Les Mery, MSc, Health Canada, Ottawa, Ont.
Mark D. Montgomery, MD, University of Calgary, Calgary, Alta.
Paul Pianosi, MD, Dalhousie University, Halifax, N.S.
Michelle Piwniuk, RRT, CAE, Canadian Society of Respiratory Therapists, Winnipeg, Man.
Amy Plint, MD, Canadian Association of Emergency Physicians, Ottawa, Ont.
John Joseph Reisman, MD, University of Ottawa, Ottawa, Ont.
Georges Rivard, MD, University of Laval, Quebec City, Que.
Malcolm Sears, MB, ChB, McMaster University, Hamilton, Ont.
F. Estelle R. Simons, MD, University of Manitoba, Winnipeg, Man.
Sheldon Spier, MD, University of Calgary, Calgary, Alta.
Robert L. Thivierge, MD, Universite de Montréal, Montréal, Que.
Wade Watson, MD, University of Manitoba, Winnipeg, Man.
Barry Zimmerman, MD, St. Michael's Hospital, Toronto, Ont.
†Members of the Adult Asthma Working Group of the Canadian Thoracic Society:
Tony Bai, MD, University of British Columbia, Vancouver, B.C.
Meyer Balter, MD, University of Toronto, Toronto, Ont.
Charles Bayliff, PharmD, London Health Sciences Centre, London, Ont.
Allan Becker, MD, University of Manitoba, Winnipeg, Man.
Louis-Philippe Boulet, MD, Université Laval, Sainte-Foy, Que.
Dennis Bowie, MD, Dalhousie University, Halifax, N.S.
André Cartier, MD, Université de Montréal, Montréal, Que.
Andrew Cave, MD, University of Alberta, Edmonton, Alta.
Kenneth Chapman, MD, University of Toronto, London, Ont.
Donald Cockcroft, MD, University of Saskatchewan, Saskatoon, Sask.
Robert Cowie, MD, University of Calgary, Calgary, Alta.
Stephen Coyle, MD, University of Manitoba, Winnipeg, Man.
Francine M. Ducharme, MD, McGill University, Montréal, Que.
Pierre Ernst, MD, McGill University, Montréal, Que.
Shelagh Finlayson, CAE, Ontario Lung Association, Toronto, Ont.
J. Mark FitzGerald, MD, University of British Columbia, Vancouver, B.C.
Frederick E Hargreave, MD, McMasterUniversity, Hamilton, Ont.
Donna Hogg, MS, RN, CAE, Dalhousie University, Halifax, N.S.
Alan Kaplan, MD, Richmond Hill, Ont.
Harold Kim, MD, Kitchener-Waterloo, Ont.
Cheryle Kelm, BPT, MSc, University of Calgary, Calgary, Alta.
Catherine Lemière, MD, Université de Montréal, Montréal, Que.
Paul O'Byrne, MD, McMaster University, Hamilton, Ont.
Malcolm Sears, MD, ChB, McMaster University, Hamilton, Ont.
Andrea White Markham, RRT, CAE, William Osler Health Centre, Brampton, Ont.
This article has been peer reviewed.
Competing interests: Allan Becker has received consultancy fees, speaker fees and/or grant support from all companies involved in asthma therapy in Canada. Catherine Lemière has received consultancy fees from ALTANA Pharma, AstraZeneca, GlaxoSmithKline and Merck Frosst; she has also received speaker fees from GlaxoSmithKline. Denis Bérubé is on the advisory boards of ALTANA Pharma and GlaxoSmithKline; he has received speaker fees from ALTANA Pharma, AstaZeneca, GlaxoSmithKline, Merck Frosst and 3M Pharmaceutical and travel assistance from GlaxoSmithKline. Louis-Philippe Boulet has been on the advisory boards of ALTANA Pharma, AstraZeneca, GlaxoSmithKline, Merck Frosst and Novartis; he has received speaker fees from AstraZeneca, GlaxoSmithKline, Merck Frosst and 3M Pharmaceutical, sponsorship for basic research from ALTANA Pharma, AstraZeneca, Merck Frosst and 3M Pharmaceutical and additional funding for participation in multicentre studies of the pharmacotherapy of asthma from AstraZeneca, ALTANA Pharma, Dynavax, Genentech, GlaxoSmithKline, Merck Frosst, Novartis, Roche, Schering and 3M Pharmaceutical. Francine Ducharme has received speaker fees from Merck. Mark FitzGerald has received honoraria, research funding and/or speaker fees from ALTANA Pharma, AstraZeneca, GlaxoSmithKline, Merck Frosst, Novartis and Roche. Thomas Kovesi has received consultancy fees and travel assistance to attend meetings from Merck Sharp and Dohme and from ALTANA Canada, and he has received speaker fees from Merck Sharp and Dohme.
Correspondence to: Dr. Allan Becker, Section of Allergy and Clinical Immunology, Department of Pediatrics and Child Health, University of Manitoba AE101–820 Sherbrook St., Winnipeg MB R3A 1R9; tel 204-787-2448; fax 204-787-5040; becker@cc.umanitoba.ca
References
- 1.Hargreave FE, Dolovich J, Newhouse MT. The assessment and treatment of asthma: a conference report. J Allergy Clin Immunol 1990;85(6):1098-111. [DOI] [PubMed]
- 2.Ernst P, Fitzgerald J, Spier S. Canadian Asthma Consensus Conference: summary of recommendations. Can Respir J 1996;3:89-100.
- 3.Boulet LP, Becker A, Bérubé D, Beveridge R, Ernst P. Canadian asthma consensus report, 1999. CMAJ 1999;161(11 suppl):S1-62. [PMC free article] [PubMed]
- 4.Boulet LP, Bai T, Becker A, Bérubé D, Beveridge R, Bowie DM, et al. What is new since the last (1999) Canadian Asthma Consensus Guidelines? Can Respir J 2001;8(suppl A):5-27A. [DOI] [PubMed]
- 5.Steering committee on clinical practice guidelines for the care and treatment of breast cancer: a Canadian consensus document. CMAJ 1998;158(3 suppl):S1-2. [PubMed]
- 6.Lemiere C, Bai T, Balter M, Bayliff C, Becker A, Boulet LP, et al. Adult asthma consensus guidelines update 2003. Can Respir J 2004;11(suppl A):9-18A. [DOI] [PubMed]
- 7.Palmer LJ, Rye PJ, Gibson NA, Burton PR, Landau LI, Lesouef PN. Airway responsiveness in early infancy predicts asthma, lung function, and respiratory symptoms by school age. Am J Respir Crit Care Med 2001;163(1):37-42. [DOI] [PubMed]
- 8.Delacourt C, Benoist MR, Waernessyckle S, Rufin P, Brouard JJ, deBlic J, et al. Relationship between bronchial responsiveness and clinical evolution in infants who wheeze: a four-year prospective study. Am J Respir Crit Care Med 2001;164(8 pt 1):1382-6. [DOI] [PubMed]
- 9.Godfrey S. Ups and downs of nitric oxide in chesty children. Am J Respir Crit Care Med 2002;166(4):438-9. [DOI] [PubMed]
- 10.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. N Engl J Med 1995;332(3):133-8. [DOI] [PubMed]
- 11.Stein RT, Holberg CJ, Morgan WJ, Wright AL, Lombardi E, Taussig L, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax 1997;52(11):946-52. [DOI] [PMC free article] [PubMed]
- 12.Van Asperen PP, Mukhi A. Role of atopy in the natural history of wheeze and bronchial hyper-responsiveness in childhood. Pediatr Allergy Immunol 1994;5:178-83. [DOI] [PubMed]
- 13.Lau S, Nickel R, Niggemann B, Gruber C, Sommerfeld C, Illi S, et al. The development of childhood asthma: lessons from the German Multicentre Allergy Study (MAS). Paediatr Respir Rev 2002;3(3):265-72. [DOI] [PubMed]
- 14.Rhodes HL, Thomas P, Sporik R, Holgate ST, Cogswell JJ. A birth cohort study of subjects at risk of atopy: twenty-two-year follow-up of wheeze and atopic status. Am J Respir Crit Care Med 2002;165(2):176-80. [DOI] [PubMed]
- 15.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000;162(4 pt 1):1403-6. [DOI] [PubMed]
- 16.Reijonen TM, Kotaniemi-Syrjanen A, Korhonen K, Korppi M. Predictors of asthma three years after hospital admission for wheezing in infancy. Pediatrics 2000;106(6):1406-12. [DOI] [PubMed]
- 17.Duff AL, Pomeranz ES, Gelber LE, Price GW, Farris H, Hayden FG, et al. Risk factors for acute wheezing in infants and children: viruses, passive smoke, and IgE antibodies to inhalant allergens. Pediatrics 1993;92(4):535-40. [PubMed]
- 18.Kotaniemi-Syrjanen A, Reijonen TM, Romppanen J, Korhonen K, Savolainen K, Korppi M. Allergen-specific immunoglobulin E antibodies in wheezing infants: the risk for asthma in later childhood. Pediatrics 2003;111(3):e255-61. [DOI] [PubMed]
- 19.Koller DY, Wojnarowski C, Herkner KR, Weinlander G, Raderer M, Eichler I, et al. High levels of eosinophil cationic protein in wheezing infants predict the development of asthma. J Allergy Clin Immunol 1997;99(6 pt 1):752-6. [DOI] [PubMed]
- 20.Shields MD, Brown V, Stevenson EC, Fitch PS, Schock BC, Turner G, et al. Serum eosinophilic cationic protein and blood eosinophil counts for the prediction of the presence of airways inflammation in children with wheezing. Clin Exp Allergy 1999;29(10):1382-9. [DOI] [PubMed]
- 21.Svanes C, Jarvis D, Chinn S, Burney P. Childhood environment and adult atopy: results from the European Community Respiratory Health Survey. J Allergy Clin Immunol 1999;103(3 pt 1):415-20. [DOI] [PubMed]
- 22.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA 2002;288(8):963-72. [DOI] [PubMed]
- 23.Apelberg BJ, Aoki Y, Jaakkola JJ. Systematic review: exposure to pets and risk of asthma and asthma-like symptoms. J Allergy Clin Immunol 2001;107(3):455-60. [DOI] [PubMed]
- 24.Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitization, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet 2001;357(9258):752-6. [DOI] [PubMed]
- 25.Remes ST, Castro-Rodriguez JA, Holberg CJ, Martinez FD, Wright AL. Dog exposure in infancy decreases the subsequent risk of frequent wheeze but not of atopy. J Allergy Clin Immunol 2001;108(4):509-15. [DOI] [PubMed]
- 26.Custovic A, Simpson BM, Simpson A, Kissen P, Woodcock A. Effect of environmental manipulation in pregnancy and early life on respiratory symptoms and atopy during first year of life: a randomised trial. Lancet 2001;358(9277):188-93. [DOI] [PubMed]
- 27.Celedon JC, Litonjua AA, Ryan L, Platts-Mills T, Weiss ST, Gold DR. Exposure to cat allergen, maternal history of asthma, and wheezing in first 5 years of life. Lancet 2002;360(9335):781-2. [DOI] [PubMed]
- 28.Gdalevich M, Mimouni D, Mimouni M. Breast-feeding and the risk of bronchial asthma in childhood: a systematic review with meta-analysis of prospective studies. J Pediatr 2001;139(2):261-6. [DOI] [PubMed]
- 29.Oddy WH, Holt PG, Sly PD, Read AW, Landau LI, Stanley FJ, et al. Association between breast feeding and asthma in 6 year old children: findings of a prospective birth cohort study. BMJ 1999;319(7213):815-9. [DOI] [PMC free article] [PubMed]
- 30.Wright AL, Holberg CJ, Taussig LM, Martinez FD. Factors influencing the relation of infant feeding to asthma and recurrent wheeze in childhood. Thorax 2001;56(3):192-7. [DOI] [PMC free article] [PubMed]
- 31.Sears MR, Greene JM, Willan AR, Taylor DR, Flannery EM, Cowan JO, et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet 2002;360(9337):901-7. [DOI] [PubMed]
- 32.Allergic factors associated with the development of asthma and the influence of cetirizine in a double-blind, randomised, placebo-controlled trial: first results of ETAC. Early Treatment of the Atopic Child. Pediatr Allergy Immunol 1998;9(3):116-24. [PubMed]
- 33.Boulet LP. Long- versus short-acting beta 2-agonists. Implications for drug therapy. Drugs 1994;47(2):207-22. [DOI] [PubMed]
- 34.Tattersfield AE, Lofdahl CG, Postma DS, Eivindson A, Schreurs AG, Rasidakis A, et al. Comparison of formoterol and terbutaline for as-needed treatment of asthma: a randomised trial. Lancet 2001;357(9252):257-61. [DOI] [PubMed]
- 35.Rodrigo GJ. Inhaled therapy for acute adult asthma. Curr Opin Allergy Clin Immunol 2003;3(3):169-75. [DOI] [PubMed]
- 36.Plotnick LH, Ducharme FM. Should inhaled anticholinergics be added to beta2 agonists for treating acute childhood and adolescent asthma? A systematic review. BMJ 1998;317(7164):971-7. [DOI] [PMC free article] [PubMed]
- 37.Adams NP, Bestall JB, Jones PW. Inhaled beclomethasone versus placebo for chronic asthma [Cochrane review]. In: The Cochrane Library; Issue 1, 2003. Oxford: Update Software.
- 38.Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet 2003;361(9363):1071-6. [DOI] [PubMed]
- 39.Sharek PJ, Bergman DA. The effect of inhaled steroids on the linear growth of children with asthma: a meta-analysis. Pediatrics 2000;106(1):E8. [DOI] [PubMed]
- 40.Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000;343(15):1064-9. [DOI] [PubMed]
- 41.O'Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001;164(8 pt 1):1392-7. [DOI] [PubMed]
- 42.Knorr B, Franchi LM, Bisgaard H, Vermeulen JH, LeSouef P, Santanello N, et al. Montelukast, a leukotriene receptor antagonist, for treatment of persistent asthma in children aged 2 to 5 years. Pediatrics 2001;108(3):E48. [DOI] [PubMed]
- 43.Knorr B, Matz J, Bernstein JA, Nguyen H, Seidenberg BC, Reiss TF, et al. Montelukast for chronic asthma in 6- to 14-year-old children: a randomized, double-blind trial. JAMA 1998;279(15):1181-6. [DOI] [PubMed]
- 44.Pearlman DS, Lampl KL, Dowling PJ Jr, Miller CJ, Bonuccelli CM. Effectiveness and tolerability of zafirlukast for the treatment of asthma in children. Clin Ther 2000;22(6):732-47. [DOI] [PubMed]
- 45.Ducharme FM, Hicks GC. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and /or chronic asthma [Cochrane review]. In: The Cochrane Library; Issue 3, 2003. Oxford: Update Software. [DOI] [PubMed]
- 46.Ducharme FM. Inhaled glucocorticoids versus leukotriene receptor antagonists as single agent asthma treatment; systematic review of current evidence. BMJ 2003;326(7390):621-5. [DOI] [PMC free article] [PubMed]
- 47.Wilson NM, Silverman M. Treatment of acute, episodic asthma in preschool children using intermittent high dose inhaled steroids at home. Arch Dis Child 1990;65(4):407-10. [DOI] [PMC free article] [PubMed]
- 48.Connett G, Lenney W. Prevention of viral induced asthma attacks using inhaled budesonide. Arch Dis Child 1993;68(1):85-7. [DOI] [PMC free article] [PubMed]
- 49.Volovitz B, Nussinovitch M, Finkelstein Y, Harel L, Varsano I. Effectiveness of inhaled corticosteroids in controlling acute asthma exacerbations in children at home. Clin Pediatr (Phila) 2001;40(2):79-86. [DOI] [PubMed]
- 50.Svedmyr J, Nyberg E, Asbrink-Nilsson E, Hedlin G. Intermittent treatment with inhaled steroids for deterioration of asthma due to upper respiratory tract infections. Acta Paediatr 1995;84(8):884-8. [DOI] [PubMed]
- 51.Hedlin G, Svedmyr J, Ryden AC. Systemic effects of a short course of betamethasone compared with high-dose inhaled budesonide in early childhood asthma. Acta Paediatr 1999;88(1):48-51. [DOI] [PubMed]
- 52.Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA). BMJ 2000;320(7246):1368-73. [DOI] [PMC free article] [PubMed]
- 53.Matz J, Emmett A, Rickard K, Kalberg C. Addition of salmeterol to low-dose fluticasone versus higher-dose fluticasone: an analysis of asthma exacerbations. J Allergy Clin Immunol 2001;107(5):783-9. [DOI] [PubMed]
- 54.Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, et al. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285(20):2583-93. [DOI] [PubMed]
- 55.Ducharme FM. Anti-leukotrienes as add-on therapy to inhaled glucocorticoids in patients with asthma: systematic review of current evidence [review]. BMJ 2002;324(7353):1545. [DOI] [PMC free article] [PubMed]
- 56.Yurdakul AS, Calisir HC, Tunctan B, Ogretensoy M. Comparison of second controller medications in addition to inhaled corticosteroid in patients with moderate asthma. Respir Med 2002;96(5):322-9. [DOI] [PubMed]
- 57.Bjermer L, Bisgaard H, Bousquet J, Fabbri LM, Greening A, Haahtela T, et al. Montelukast or salmeterol combined with an inhaled steroid in adult asthma: design and rationale of a randomized, double-blind comparative study (the IMPACT Investigation of Montelukast as a Partner Agent for Complementary Therapy-trial). Respir Med 2000;94(6):612-21. [DOI] [PubMed]
- 58.Fish JE, Israel E, Murray JJ, Emmett A, Boone R, Yancey SW, et al. Salmeterol powder provides significantly better benefit than montelukast in asthmatic patients receiving concomitant inhaled corticosteroid therapy. Chest 2001;120(2):423-30. [DOI] [PubMed]
- 59.Ringdal N, Eliraz A, Pruzinec R, Weber HH, Mulder PG, Akveld M, et al. The salmeterol/fluticasone combination is more effective than fluticasone plus oral montelukast in asthma. Respir Med 2003;97(3):234-41. [DOI] [PubMed]
- 60.Kamps AW, Brand PL, Roorda RJ. Determinants of correct inhalational technique in children attending a hospital-based asthma clinic. Acta Paediatr 2002;91(2):159-63. [DOI] [PubMed]
- 61.Kamps AW, van Ewijk B, Roorda RJ, Brand PL. Poor inhalation technique, even after inhalation instructions, in children with asthma. Pediatr Pulmonol 2000;29(1):39-42. [DOI] [PubMed]
- 62.Chen SH, Yin TJ, Huang JL. An exploration of the skills needed for inhalation therapy in schoolchildren with asthma in Taiwan. Ann Allergy Asthma Immunol 2002;89(3):311-5. [DOI] [PubMed]
- 63.Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J 2002;19(2):246-51. [DOI] [PubMed]
- 64.Lenney J, Innes JA, Crompton GK. Inappropriate inhaler use: assessment of use and patient preference of seven inhalation devices. EDICI. Respir Med 2000;94(5):496-500. [DOI] [PubMed]
- 65.Hilton S. An audit of inhaler technique among asthma patients of 34 general practitioners. Br J Gen Pract 1990;40(341):505-6. [PMC free article] [PubMed]
- 66.Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess 2001;5:1-149. [DOI] [PubMed]
- 67.Delgado A, Chou KJ, Silver EJ, Crain EF. Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med 2003;157:76-80. [DOI] [PubMed]
- 68.Mandelberg A, Tsehori S, Houri S, Gilad E, Morag B, Priel IE. Is nebulized aerosol treatment necessary in the pediatric emergency department? Chest 2000;117:1309-13. [DOI] [PubMed]
- 69.Cates CJ, Rowe BH, Bara A. Holding chambers versus nebulizers for beta agonist treatment of acute asthma [Cochrane review]. In: The Cochrane Library; Issue 2, 2002. Oxford: Update Software. [DOI] [PubMed]
- 70.Closa RM, Ceballos JM, Gomez-Papi A, Galiana AS, Gutierrez C, Marti-Henneber C. Efficacy of bronchodilators administered by nebulizers versus spacer devices in infants with acute wheezing. Pediatr Pulmonol 1998;26:344-8. [DOI] [PubMed]
- 71.Rubilar L, Castro-Rodriguez JA, Girardi G. Randomized trial of salbutamol via metered-dose inhaler with spacer versus nebulizer for acute wheezing in children less than 2 years of age. Pediatr Pulmonol 2000;29:264-9. [DOI] [PubMed]
- 72.Chua HL, Collis GG, Newbury AM, Chan K, Bower GD, Sly PD, et al. The influence of age on aerosol deposition in children with cystic fibrosis. Eur Respir J 1994;7:2185-91. [DOI] [PubMed]
- 73.Agertoft L, Pedersen S. Importance of training for correct Turbuhaler use in preschool children. Acta Paediatr 1998;87:842-7. [DOI] [PubMed]
- 74.Goren A, Noviski N, Avital A, Maayan C, Stahl E, Godfrey S, et al. Assessment of the ability of young children to use a powder inhaler device (Turbuhaler). Pediatr Pulmonol 1994;18:77-80. [DOI] [PubMed]
- 75.Drblik S, Lapierre G, Thivierge R, Turgeon J, Gaudreault P, Cummins-McManus B, et al. Comparative efficacy of terbutaline sulphate delivered by Turbuhaler dry powder inhaler or pressurised metered dose inhaler with Nebuhaler spacer in children during an acute asthmatic episode. Arch Dis Child 2003;88:319-23. [DOI] [PMC free article] [PubMed]
- 76.van der PJ. Klein JJ, van Herwaarden CL, Zielhuis GA, Seydel ER. Multiple inhalers confuse asthma patients. Eur Respir J 1999;14:1034-7. [DOI] [PubMed]
- 77.Tal A, Golan H, Grauer N, Aviram M, Albin D, Quastel MR. Deposition pattern of radiolabeled salbutamol inhaled from a metered-dose inhaler by means of a spacer with mask in young children with airway obstruction. J Pediatr 1996;128(4):479-84. [DOI] [PubMed]
- 78.Onhoj J, Thorsson L, Bisgaard H. Lung deposition of inhaled drugs increases with age. Am J Respir Crit Care Med 2000;162(5):1819-22. [DOI] [PubMed]
- 79.Allergen immunotherapy: therapeutic vaccines for allergic diseases. Geneva: January 27-29 1997. Allergy 1998;53(44 suppl):1-42. [DOI] [PubMed]
- 80.Sigman K, Mazer B. Immunotherapy for childhood asthma: is there a rationale for its use? Ann Allergy Asthma Immunol 1996;76(4):299-309. [DOI] [PubMed]
- 81.Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy 2001;31(9):1392-7. [DOI] [PubMed]
- 82.Gibson PG, Powell H, Coughlan J, Wilson AJ, Abramson M, Haywood P, et al. Self-management education and regular practitioner review for adults with asthma [Cochrane review]. In: The Cochrane Library; Issue 1, 2003. Oxford: Update Software. [DOI] [PubMed]
- 83.FitzGerald JM, Turner MO. Delivering asthma education to special high risk groups. Patient Educ Couns 1997;32(1 suppl):S77-86. [DOI] [PubMed]
- 84.Cote J, Bowie DM, Robichaud P, Parent JG, Battisti L, Boulet LP. Evaluation of two different educational interventions for adult patients consulting with an acute asthma exacerbation. Am J Respir Crit Care Med 2001;163(6):1415-9. [DOI] [PubMed]
- 85.Yilmaz A, Akkaya E. Evaluation of long-term efficacy of an asthma education programme in an out-patient clinic. Respir Med 2002;96(7):519-24. [DOI] [PubMed]
- 86.Couturaud F, Proust A, Frachon I, Dewitte JD, Oger E, Quiot JJ, et al. Education and self-management: a one-year randomized trial in stable adult asthmatic patients. J Asthma 2002;39(6):493-500. [DOI] [PubMed]
- 87.Put C, van den Bergh O, Lemaigre V, Demedts M, Verleden G. Evaluation of an individualised asthma programme directed at behavioural change. Eur Respir J 2003;21(1):109-15. [DOI] [PubMed]
- 88.Osman LM, Calder C, Godden DJ, Friend JA, McKenzie L, Legge JS, et al. A randomised trial of self-management planning for adult patients admitted to hospital with acute asthma. Thorax 2002;57(10):869-74. [DOI] [PMC free article] [PubMed]
- 89.Thoonen BP, Schermer TR, Van Den BG, Molema J, Folgering H, Akkermans RP et al. Self-management of asthma in general practice, asthma control and quality of life: a randomised controlled trial. Thorax 2003; 58(1):30-36. [DOI] [PMC free article] [PubMed]
- 90.Cote J, Cartier A, Robichaud P, Boutin H, Malo JL, Rouleau M, et al. Influence of asthma education on asthma severity, quality of life and environmental control. Can Respir J 2000;7(5):395-400. [DOI] [PubMed]
- 91.Ignacio-Garcia JM, Pinto-Tenorio M, Chocron-Giraldez MJ, Cabello-Rueda F, Lopez-Cozar Gil AI, Ignacio-Garcia JM, et al. Benefits at 3 yrs of an asthma education programme coupled with regular reinforcement. Eur Respir J 2002;20(5):1095-101. [DOI] [PubMed]
- 92.Cowie RL, Underwood MF, Little CB, Mitchell I, Spier S, Ford GT. Asthma in adolescents: a randomized, controlled trial of an asthma program for adolescents and young adults with severe asthma. Can Respir J 2002;9(4):253-9. [DOI] [PubMed]
- 93.Shah S, Peat JK, Mazurski EJ, Wang H, Sindhusake D, Bruce C, et al. Effect of a peer led programme for asthma education in adolescents: cluster randomized controlled trial. BMJ 2001;32(7286)2:583-5. [DOI] [PMC free article] [PubMed]
- 94.Guevara JP, Wolf FM, Grum CM, Clark NM. Effects of educational interventions for self management of asthma in children and adolescents: systematic review and meta-analysis. BMJ 2003;326(7402):1308-9. [DOI] [PMC free article] [PubMed]
- 95.Bonner S, Zimmerman BJ, Evans D, Irigoyen M, Resnick D, Mellins RB. An individualized intervention to improve asthma management among urban Latino and African-American families. J Asthma 2002;39(2):167-79. [DOI] [PubMed]
- 96.Moe EL, Eisenberg JD, Vollmer WM, Wall MA, Stevens VJ, Hollis JF. Implementation of “Open Airways” as an educational intervention for children with asthma in an HMO. J Pediatr Health Care 1992;6(5 pt 1):251-5. [DOI] [PubMed]
- 97.Bartholomew LK, Gold RS, Parcel GS, Czyzewski DI, Sockrider MM, Fernandez M, et al. Watch, discover, think, and act: evaluation of computer-assisted instruction to improve asthma self-management in inner-city children. Patient Educ Couns 2000;39(2-3):269-80. [DOI] [PubMed]
- 98.Chapman KR, Ernst P, Grenville A, Dewland P, Zimmerman S. Control of asthma in Canada: failure to achieve guideline targets. Can Respir J 2001; 8:35A-40A. [DOI] [PubMed]


