Abstract
Epidemiological studies suggest H2O2-producing lactobacilli protect women against gonorrhea. Here we demonstrate that Lactobacillus crispatus and Lactobacillus jensenii, the most common lactobacilli in the female genital tract, inhibit gonococci in both acidic and neutral pH conditions. Inhibition was neutralized by bovine catalase, suggesting that H2O2 is the primary mediator of inhibition.
Neisseria gonorrhoeae has a major impact on health worldwide, with the highest morbidity and mortality occurring in females. The most common site of gonococcal infection in females of reproductive age is the endocervix (9). A variety of host factors may contribute to the success or failure of N. gonorrhoeae to infect this site, including the types of commensal flora that inhabit the lower genital tract. Lactobacilli, the most common facultatively anaerobic bacteria of the vagina (13) and endocervix (7), play an important role in maintaining a normal vaginal ecosystem through the production of organic acids, bacteriocins, and hydrogen peroxide, all of which may protect against pathogens (13). Among the many microbes that inhibit N. gonorrhoeae in vitro (2, 8, 15, 16), lactobacilli are of particular interest due to reported associations between a reduced risk of gonorrhea and colonization by lactobacilli (1, 10, 15).
Lactobacillus crispatus and Lactobacillus jensenii are the predominant Lactobacillus spp. in the female lower genital tract. Lactobacillus acidophilus and Lactobacillus gasseri are also frequently isolated (1, 4, 6, 14). Inhibition of N. gonorrhoeae in vitro has been reported only for L. acidophilus (20), however, and for unidentified H2O2-producing clinical isolates of lactobacilli (15). The capacity of the predominant Lactobacillus spp. of the genital tract to inhibit N. gonorrhoeae is therefore unclear. Here we tested H2O2-producing strains of L. crispatus, L. jensenii, L. gasseri, and L. acidophilus for the capacity to inhibit two gonococcal laboratory strains (MS11 and FA1090) that are infectious in male volunteers (3, 18) and four clinical isolates of N. gonorrhoeae (Table 1) using a modified version of the agar overlay technique of Saigh et al. (15). Briefly, saline suspensions containing ca. 108 CFU of lactobacilli harvested from lactobacillus-MRS agar plates per ml were prepared. Fifty-microliter samples of the suspensions were inoculated onto heart infusion agar (HIA) that was adjusted to the desired pH (range, 5.8 to 7.6) prior to autoclaving. After 20 to 24 h of incubation, 7.5 ml of GC agar were poured onto the HIA plates and allowed to solidify. Suspensions (100 μl) containing ca. 106 CFU of the Neisseria species or Escherichia coli strains to be tested (target organisms) were spread onto the agar overlay and incubated for 20 to 24 h. The presence of a zone of growth inhibition around the target strain was considered positive for inhibition. For all experiments, the number of CFU in the lactobacillus and target cell suspensions was confirmed by standard serial dilution and culture. Growth of lactobacilli on HIA did not appreciably change the pH of the agar as determined by the use of pH indicators (data not shown). All media were purchased from Difco Laboratories (Detroit, Mich.). All incubations were at 37°C in 5% CO2.
TABLE 1.
Bacterial strains used in this study
| Bacterial strain | Description | Source |
|---|---|---|
| N. gonorrhoeae | ||
| FA1090 | Laboratory strain | Our laboratory |
| MS11 | Laboratory strain | H. S. Seiferta |
| 400 | Clinical isolate | H. S. Seifent |
| 644 | Clinical isolate | H. S. Seifert |
| 229 | Clinical isolate | H. S. Seifert |
| 555 | Clinical isolate | H. S. Seifert |
| N. cinerea 14685 | Commensal species | ATCCb |
| Lactobacillus spp. | ||
| L. acidophilus 4356 | Human isolate | ATCC |
| L. crispatus 33197 | Human urine isolate | ATCC |
| L. jensenii 25258 | Human vaginal isolate | ATCC |
| L. gasseri 33323 | ATCC | |
| E. coli | ||
| EFC-9 | Fecal isolate | H. L. T. Mobleyc |
| EFC-10 | Fecal isolate | H. L. T. Mobley |
| J96 | Uropathogenic strain | A. D. O'Briend |
Northwestern University, Evanston, Ill.
ATCC, American Type Culture Collection, Manassas, Va.
University of Maryland, Baltimore, Md.
Uniformed Services University of Health Sciences, Bethesda, Md.
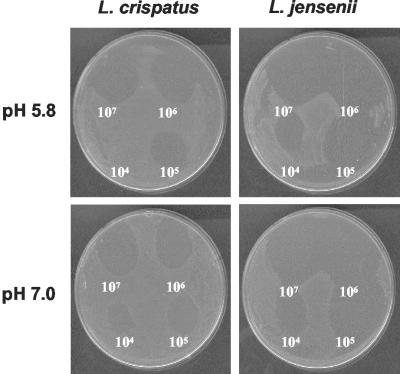
All four lactobacillus strains inhibited all gonococcal strains tested at low pH; only L. jensenii and L. crispatus inhibited N. gonorrhoeae at neutral pH. None of the lactobacilli inhibited E. coli, and only L. jensenii inhibited Neisseria cinerea, a commensal organism of the respiratory and genital tracts (Table 2). Serial dilution of the lactobacillus suspensions before inoculating the base agar resulted in visibly fewer lactobacilli within the inoculated region. On the basis of this semiquantitative evaluation of the number of lactobacilli present during the assay, L. jensenii consistently demonstrated higher levels of inhibition against N. gonorrhoeae than the other three lactobacillus strains (Fig. 1). Inoculation of the overlay agar with >106 CFU of N. gonorrhoeae significantly reduced the zones of inhibition and reproducibility of the assay (D. J. Kuch and A. E. Jerse, unpublished observations).
TABLE 2.
Inhibition of N. gonorrhoeae by H2O2-producing Lactobacillus spp.a of the female genital tract under conditions of acidic versus neutral pH
| Organism | Inhibition of organism by lactobacillib
|
|||||||
|---|---|---|---|---|---|---|---|---|
| pH 5.8
|
pH 7.2
|
|||||||
| LA | LC | LJ | LG | LA | LC | LJ | LG | |
| N. gonorrhoeae | ||||||||
| FA1090 | + | + | + | + | − | + | + | − |
| MS11 | + | + | + | +/− | − | + | + | − |
| 229 | + | + | + | + | − | + | + | − |
| 397 | + | + | + | + | − | + | + | − |
| 400 | + | + | + | + | − | + | + | − |
| 644 | + | + | + | + | − | + | + | − |
| N. cinerea | − | − | + | − | − | − | + | − |
| E. coli | ||||||||
| EFC-9 | − | − | − | − | − | − | − | − |
| EFC-15 | − | − | − | − | − | − | − | − |
| J96 | − | − | − | − | − | − | − | − |
All Lactobacillus spp. produced H2O2 as detected in a qualitative H2O2 assay by McGroarty et al. (11).
Abbreviations: LA, L. acidophilus; LC, L. crispatus; LJ, L. jensenii; LG, L. gasseri. Symbols: +, inhibition; −, absence of inhibition; +/−, weak inhibition.
FIG. 1.
Inhibition of N. gonorrhoeae strain FA1090 by L. crispatus and L. jensenii when cultured at acidic or neutral pH as detected in an agar overlay assay. An undiluted suspension containing 107 CFU of lactobacilli was inoculated onto the upper right quadrant, and 10-fold serial dilutions of this suspension were inoculated in a clockwise direction onto the base agar the day before inoculating with N. gonorrhoeae.
The primary mediator of inhibition in all four strains appeared to be H2O2 based on the ability to neutralize inhibition by incorporating bovine catalase (Worthington Biochemicals, Lakewood, N.J.) into the overlay medium. Inhibition of N. gonorrhoeae by L. crispatus and L. jensenii when cultured at pH 7.0 was neutralized by 5 U of bovine catalase per ml. At an acidic pH, a 10-fold-higher concentration of catalase was required to neutralize inhibition by L. jensenii, and 100-fold-more catalase was required to neutralize inhibition by L. acidophilus and L. crispatus (Table 3). This result was reproducible, although it appears to be inconsistent with the large inhibitory zones produced by L. jensenii compared to those produced by L. crispatus (Fig. 1). In general, more catalase was required to neutralize the inhibition by all lactobacilli as the pH of the base agar decreased (pH 7.6, 7.0, 6.6, 6.3, and 5.8) (data not shown). This observation may be explained by increased production of H2O2 by lactobacilli at low pH or increased stability of H2O2 at low pH (5).
TABLE 3.
Effect of bovine catalase on inhibition of N. gonorrhoeae strain FA1090 by lactobacilli or increasing concentrations of H2O2
| pH, lactobacillus, and/or H2O2 concn | Effecta of bovine catalase concnb in overlay agar on inhibition
|
||||
|---|---|---|---|---|---|
| 0 U | 0.5 U | 5 U | 50 U | 500 U | |
| Base agar pH 7.4 | |||||
| L. crispatus | + | + | − | − | − |
| L. jensenii | + | + | − | − | − |
| 18 mM H2O2 | + | − | − | − | − |
| 50 mM H2O2 | + | + | − | − | − |
| 100 mM H2O2 | + | + | − | − | − |
| Base agar pH 5.8 | |||||
| L. acidophilus | + | + | + | + | − |
| L. crispatus | + | + | + | + | − |
| L. jensenii | + | + | + | − | − |
| L. gasseri | + | + | + | − | − |
| 18 mM H2O2 | + | − | − | − | − |
| 50 mM H2O2 | + | + | − | − | − |
| 100 mM H2O2 | + | + | − | − | − |
Symbols: +, inhibition; −, absence of inhibition.
Catalase concentration is expressed in units per mg of protein (1 U decomposes 1 μmol of H2O2 per min at 25°C and pH 7.0).
These data support the hypothesis that commensal lactobacilli in the lower genital tract reduce the risk of gonococcal infection in women through the production of H2O2. It is not known, however, if lactobacilli produce sufficient amounts of H2O2 in the low-oxygen-tension environment of the lower genital tract (12, 19) for inhibition of N. gonorrhoeae to occur. Also, theoretically, gonococcal catalase should defend against H2O2-producing lactobacilli by breaking H2O2 down to water and molecular oxygen. The inability of gonococcal catalase to neutralize H2O2-mediated lactobacillus inhibition in vitro could be due to the production of overwhelming amounts of H2O2 or to a lactobacillus-encoded factor that interferes with the ability of gonococcal catalase to cleave H2O2.
The relationship between culture pH and the relative inhibitory potential of each Lactobacillus species tested is intriguing in light of the cyclical change in pH of the female lower genital tract. The average pH values of vaginal and cervical mucus during the proliferative stage of the menstrual cycle are 4.6 (range, 3.3 to 7.4) and 6.8 (range, 5.5 to 8), respectively. A lower pH occurs in the luteal stage, with an average vaginal pH of 4.4 (range, 3.6 to 6.0) and endocervical pH of 6.1 (range, 5.1 to 8.4) (17). On the basis of these data, one might hypothesize that the capacity of commensal lactobacilli to protect women against gonorrhea may depend on both the species and stage of the menstrual cycle. The capacity of L. jensenii or L. crispatus to inhibit N. gonorrhoeae at both low and neutral pHs suggests that these strains may protect against gonorrhea more effectively than the other species tested.
Acknowledgments
We thank Afrin Begum for technical assistance and David Kuch for helpful reading of the manuscript.
This work was supported in part by USUHS intramural grant G173-HM.
Editor: D. L. Burns
REFERENCES
- 1.Antonio, M. A., S. E. Hawes, and S. L. Hillier. 1999. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J. Infect. Dis. 180:1950-1956. [DOI] [PubMed] [Google Scholar]
- 2.Braude, A. I., L. B. Corbeil, S. Levine, J. Ito, and J. A. McCutchan. 1978. Possible influence of cyclic menstrual changes on resistance to the gonococcus, p. 328-337. In G. F. Brooks, E. C. Gotschlich, K. K. Holmes, W. D. Sawyer, and F. E. Young (ed.), Immunobiology of Neisseria gonorrhoeae. American Society for Microbiology, Washington, D.C.
- 3.Cohen, M. S., J. G. Cannon, A. E. Jerse, L. M. Charniga, S. F. Isbey, and L. G. Whicker. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532-537. [DOI] [PubMed] [Google Scholar]
- 4.Eschenbach, D. A., P. R. Davick, B. L. Williams, S. J. Klebanoff, K. Young-Smith, C. M. Critchlow, and K. K. Holmes. 1989. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J. Clin. Microbiol. 27:251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontaine, E. A., and D. Taylor-Robinson. 1990. Comparison of quantitative and qualitative methods of detecting hydrogen peroxide produced by human vaginal strains of lactobacilli. J. Appl. Bacteriol. 69:326-331. [DOI] [PubMed] [Google Scholar]
- 6.Giorgi, A., S. Torriana, F. Dellaglio, G. Bo, E. Stola, and L. Bernuzzi. 1987. Identification of vaginal lactobacilli from asymptomatic women. Microbiologica 10:377-384. [PubMed] [Google Scholar]
- 7.Gorbach, S. L., K. B. Menda, H. Thadepalli, and L. Keith. 1973. Anaerobic microflora of the cervix in healthy women. Am. J. Obstet. Gynecol. 8:1053-1055. [DOI] [PubMed] [Google Scholar]
- 8.Hipp, S. S., W. D. Lawton, N. C. Chen, and H. A. Gaafar. 1974. Inhibition of Neisseria gonorrhoeae by a factor produced by Candida albicans. Appl. Microbiol. 27:192-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hook, E. W., and H. H. Handsfield. 1999. Gonococcal infections in the adult, p. 451-472. In K. K. Holmes, P. A. Mardh, P. F. Sparling, S. M. Lemon, W. E. Stamm, P. Piot, and J. N. Wasserheit (ed.), Sexually transmitted diseases, 3rd ed. McGraw-Hill Companies, Inc., New York, N.Y.
- 10.Martin, H. L., B. A. Richardson, P. M. Nyange, L. Lavreys, S. L. Hillier, B. Chohan, K. Mandaliya, J. O. Ndinya-Achola, J. Bwayo, and J. Kreiss. 1999. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J. Infect. Dis. 180:1863-1868. [DOI] [PubMed] [Google Scholar]
- 11.McGroarty, J. A., L. Tomeczek, D. G. Pond, G. Reid, and A. W. Bruce. 1992. Hydrogen peroxide production by lactobacillus species: correlation with susceptibility to the spermicidal compound nonoxynol-9. J. Infect. Dis. 165:1142-1144. [DOI] [PubMed] [Google Scholar]
- 12.Rashad, A. L., W. L. Toffler, N. Wolf, K. Thornburg, E. P. Kirk, G. Ellis, and W. E. Whitehead. 1992. Vaginal PO2 in healthy women and in women infected with Trichomonas vaginalis: potential implications for metronidazole therapy. Am. J. Obstet. Gynecol. 166:620-624. [DOI] [PubMed] [Google Scholar]
- 13.Redondo-Lopez, V., R. L. Cook, and J. D. Sobel. 1990. Emerging role of lactobacilli in the control and maintenance of the vaginal microflora. Rev. Infect. Dis. 12:856-872. [DOI] [PubMed] [Google Scholar]
- 14.Reid, G., J. A. McGroarty, L. Tomeczek, and A. W. Bruce. 1996. Identification and plasmid profiles of Lactobacillus species from the vagina of 100 healthy women. FEMS Immunol. Med. Microbiol. 15:23-26. [DOI] [PubMed] [Google Scholar]
- 15.Saigh, J. H., C. C. Sanders, and W. E. Sanders. 1978. Inhibition of Neisseria gonorrhoeae by aerobic and facultatively anaerobic components of the endocervical flora: evidence for a protective effect against infection. Infect. Immun. 19:704-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shtibel, R. 1976. Inhibition of growth of Neisseria gonorrhoeae by bacterial interference. Can. J. Microbiol. 22:1430-1436. [DOI] [PubMed] [Google Scholar]
- 17.Singer, A. 1975. The uterine cervix from adolescence to the menopause. Br. J. Obstet. Gynaecol. 82:81-99. [DOI] [PubMed] [Google Scholar]
- 18.Swanson, J., K. Robbins, O. Barrera, D. Corwin, J. Boslego, J. Ciak, M. Blake, and J. M. Koomey. 1987. Gonococcal pilin variants in experimental gonorrhea. J. Exp. Med. 165:1344-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner, G., and R. Levin. 1978. Oxygen tension of the vaginal surface during sexual stimulation in the human. Fertil. Steril. 30:50-53. [DOI] [PubMed] [Google Scholar]
- 20.Zheng, H., T. M. Alcorn, and M. S. Cohen. 1994. Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. J. Infect. Dis. 170:1209-1215. [DOI] [PubMed] [Google Scholar]