Abstract
The cytokine profile produced by peripheral blood mononuclear cells (PBMC) in response to leishmania antigens and the ability of interleukin-10 (IL-10) and transforming growth factor β (TGF-β) to modulate the immune response were evaluated in 21 mucosal leishmaniasis patients. Patients with mucosal disease exhibited increased gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) secretion and decreased IL-10 secretion compared to patients with classical cutaneous leishmaniasis. CD4+ Th1 cells were the main source of IFN-γ and TNF-α production in mucosal leishmaniasis patients. Evaluation of cytokine gene expression in PBMC of these patients showed that there was strong up-regulation of IFN-γ transcripts upon stimulation with leishmania antigen, in contrast to the baseline levels of IL-10 mRNA. IL-10 suppressed IFN-γ production by 48% in cell cultures from cutaneous leishmaniasis patients and by 86% in cell cultures from healthy subjects stimulated with purified protein derivative, whereas in similar conditions IL-10 suppressed IFN-γ production by 19% in cell cultures from mucosal leishmaniasis patients stimulated with leishmania antigen. TGF-β suppressed IFN-γ levels to a greater extent in healthy subjects than in mucosal leishmaniasis and cutaneous leishmaniasis patients. These data indicate that a poorly modulated T-cell response in mucosal leishmaniasis patients leads to production of high levels of proinflammatory cytokines, such as IFN-γ and TNF-α, as well as a decreased ability of IL-10 and TGF-β to modulate this response. These abnormalities may be the basis for the pathological findings observed in this disease.
Leishmania spp. are obligately intracellular parasites of macrophages that cause a spectrum of human diseases, including self-healing skin lesions, diffuse cutaneous leishmaniasis, localized cutaneous leishmaniasis (CL), mucosal leishmaniasis (ML), and visceral leishmaniasis (VL) (24). In all clinical forms, recovery and resistance to disease are dependent on T-cell responses (11, 14). For example, VL and diffuse cutaneous leishmaniasis are characterized by selective anergy of the specific cell-mediated immunity, which results in extensive involvement of the spleen and liver in the former disease and the skin in the latter disease, and abundant parasites are observed inside macrophages (12, 14).
CL is the most common form of tegumentary leishmaniasis. The hallmark of this illness is the development of single or multiple ulcerated dermal lesions, which usually promptly respond to antimonial therapy. A fraction (3%) of CL patients develop mucosal disease either at the time of ulceration or several months thereafter. ML is severely disfiguring and is characterized by exacerbated cell-mediated immunity to the microorganism and destructive lesions of the oral and nasopharyngeal cavities (13, 15). Affected individuals are often unresponsive to standard treatment and present strong intradermal skin tests and lymphocyte proliferative responses to leishmania antigens (12, 16). On the other hand, microscopic analysis of lesion biopsies reveals an intense inflammatory reaction, in which lymphocytes and macrophages predominate and few or no parasites can be found (6).
The exact pathogenic mechanism of ML has not been established. While it has been suggested that ML may represent a polar hypersensitivity reaction to leishmania infection (1), other studies have revealed a mixture of type 1 and type 2 cytokines in lesions with up-regulation of mRNA for gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin-4 (IL-4), and IL-10 (8, 27). Additionally, it has been shown that lymphocytes from ML patients produce high levels of IFN-γ and TNF-α in response to antigenic stimulation (12, 29). These data raise the question of whether immunological hyperactivity plays a part in ML. If it does, an exacerbated and unmodulated immunological response may ultimately lead to damage of host tissues. TNF-α is a proinflammatory cytokine secreted by macrophages and lymphocytes, whose production is up-regulated by IFN-γ (37). A role for TNF-α in the pathogenesis of this and other chronic inflammatory parasitic and bacterial diseases is strongly suggested by (i) increased levels of TNF-α in sera and supernatants of lymphocyte cultures (14, 29), (ii) high levels of TNF-α expression in mucosal tissue (27), (iii) decreased TNF-α levels after therapy for ML (17), and (iv) an association of TNF-α levels with cerebral malaria (19) and tissue damage following erythema nodosum leprosum (32).
In the present study, we demonstrated that an exaggerated immune response to leishmania antigens plays a role in ML, which is chiefly mediated by Th1-type CD4+ T lymphocytes and which is poorly modulated by the regulatory cytokines transforming growth factor β (TGF-β) and IL-10.
MATERIALS AND METHODS
Patients.
Twenty-one ML patients recruited at the health post of Corte de Pedra, Bahia, Brazil, were enrolled in this study. The criteria for diagnosis were a clinical picture compatible with ML, a positive delayed-type hypersensitivity response to leishmania antigens, and parasite isolation or histopathological findings characteristic of ML. All patients had a previous diagnosis of CL and were evaluated before therapy for mucosal disease. Six patients were evaluated before and 120 days after antimonial therapy (Glucantime; Rhodia, São Paulo, Brazil), at which time they were clinically cured of disease symptoms and the lesions had become scars. In order to evaluate the immunological responses of the 21 patients with ML, we matched these individuals by age and sex with 21 patients with cutaneous disease. Immunomodulation of antigen T-cell responses by IL-10 and TGF-β was evaluated in ML patients and compared to immunomodulation in CL patients. We also evaluated the role of these cytokines in modulating the immune response to a different antigen. To do this, we added IL-10 and TGF-β to cultures of cells stimulated with purified protein derivative (PPD) in PPD-skin-test-positive individuals. This research was conducted with approval of the Ethical Committee of the Hospital Universitário Prof. Edgard Santos, and informed consent was obtained from each participant.
Soluble leishmania antigen.
The leishmania lysate (crude antigen) used for cytokine production was prepared from a Leishmania amazonensis strain (MHOM-BR-86 BA-125) as previously described (12). L. amazonensis was chosen for antigen preparation because it is easy to grow and maintain and because the L. amazonensis antigen produces an immune response remarkably similar to that of Leishmania braziliensis soluble antigen. This antigen preparation was negative for endotoxin, and it did not stimulate cells from healthy subjects who were not exposed to leishmania infection.
Evaluation of cytokine protein and mRNA expression.
Peripheral blood mononuclear cells (PBMC) were separated from heparinized venous blood over a Ficoll-Hypaque gradient (Organon Teknika, Durham, N.C.), washed, and resuspended in RPMI 1640 medium (Life Technologies Gibco BRL, Grand Island, N.Y.) supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% heat-inactivated pooled human AB serum (complete medium) at a concentration of 3 × 106 cells/ml. These cells were plated in 24-well plates (catalog no. 3047; Falcon, Becton Dickinson, Lincoln Park, N.J.) and either maintained unstimulated or stimulated with L. amazonensis antigen (10 μg/ml) or PPD (2 μg/well). After incubation for 72 h at 37°C in the presence of 5% CO2, the supernatants were collected and stored frozen until cytokine determination. To evaluate the roles of IL-10 (DNAX Institute, Palo Alto, Calif.) and TGF-β (R&D Systems, Minneapolis, Minn.), these cytokines were added to the cultures at concentrations ranging from 0.5 to 20 ng/ml.
To measure IFN-γ, TNF-α, and IL-5 levels, an enzyme-linked immunosorbent assay (ELISA) sandwich technique was used (Duo-set; Genzyme, Cambridge, Mass., and Pharmingen, San Diego, Calif.). The IL-10 levels were measured by using a kit obtained from R&D Systems. In order to evaluate IL-10 and IFN-γ gene expression, PBMC from three ML patients were cultured in triplicate as described above and stimulated with leishmania antigen, medium, or phytohemagglutinin (PHA) for 72 h. Cells were harvested, mRNA was extracted, and reverse transcription (RT)-PCR were performed with the following primer pairs: for IL-10, AGG GCA CCC AGT CTG AGA ACA and CGG CCT TGC TCT TGT TTT CAC; and for IFN-γ, CAG CTC TGC ATC GTT TTG GGT TCT and TGC TCT TCG ACCG TCAAA CAG CAT. Amplicons were resolved by 1% agarose gel electrophoresis and were photographed by using a UVP apparatus (UUP Inc., Upland, Calif.) under UV light with ethidium bromide staining. Expression of hypoxanthine phosphoribosyltransferase was evaluated in parallel (with primers GCG TCG TGA TTA GTG ATG ATG AAC and TGC TCT TCG ACC TCG AAA CAG CAT) and was used for proper normalization of sample loads in analytic runs.
Identification of cytokine-producing cells.
The cellular sources of IFN-γ and TNF-α in ML were initially determined by cell removal assays. Specific subpopulations were removed from patient PBMC by different techniques. Magnetic separation was performed directly with PBMC by using specific MACS microbeads for enrichment of CD4+, CD8+, and NK cell subpopulations. To separate NK cells, we used an NK cell isolation kit (MiniMACS; Miltenyi Biotec, Sunnyvale, Calif.), which removed CD3+, CD4+, CD19+, and CD33+ cells. The percentages of contamination in the populations with cells removed, as measured by fluorescence-activated cell sorting, were as follows: population with CD4+ cells removed, 0.2% CD4+ cells; population with CD8+ cells removed, 2% CD8+ cells; and population with NK cells removed, 1% CD56+ cells.
Macrophages were removed by adherence to petri dishes (9). Briefly, 107 PBMC in RPMI 1640 medium supplemented with 10% fetal calf serum were incubated in plastic petri dishes (10 by 15 mm; Falcon, Becton Dickinson) for 2 h at 37°C. After a second removal step, nonadherent cells were recovered, and the purity of the cells was determined by esterase staining (the percentages of esterase-positive cells were 28% ± 3% before removal and 2% ± 0.7% after removal). The enriched subpopulations were then stimulated with L. amazonensis antigen as described above. Cytokines were measured by an ELISA as described above.
To further define the specific cell types involved in TNF-α production, single-cell cytoplasmic cytokine staining was performed. PBMC were analyzed to determine their intracellular cytokine expression patterns as described below and by Sornasse et al. (36). Briefly, 1-ml cultures containing 2.5 × 106 PBMC were grown in 24-well plates for 20 h with medium alone or with soluble leishmania antigen (final concentration, 10 μg/ml); a positive control containing anti-CD3/CD28 (2 ng of anti-CD3 per ml and 1 ng of anti-CD28 per ml) was included. During the last 4 h of incubation, Brefeldin-A (1 μg/ml) was added to the cultures, which impaired protein secretion by the Golgi complex. The cells were then harvested by using ice-cold phosphate-buffered saline plus azide, stained for surface markers (anti-CD4-Cychrome and anti-CD8-fluorescein isothiocyanate [FITC] [Caltag, Burlingame, Calif.] or anti-CD14-FITC [Pharmingen]), and fixed by using 2% formaldehyde. The fixed cells were then permeabilized with a solution of saponin and stained for 30 min at 4°C by using anti-TNF-α monoclonal antibody (Pharmingen) directly conjugated with phycoerythrin. FITC- and phycoerythrin-labeled immunoglobulin control antibodies, as well as control unstimulated PBMC, were also included in all experiments. Samples were analyzed with a FACSvantage or FACSsort (Becton Dickinson) with selection for the lymphocyte population. In all cases, 30,000 gated events were acquired for later analysis. This number of events was required due to the low frequency of positive events analyzed. Controls consisting of nonstimulated versus stimulated T-cell clones were used to standardize the antibodies used; in addition, media alone and polyclonal antibody-stimulated (anti-CD3/CD28) PBMC were used as positive controls.
Statistical analysis.
A Mann-Whitney test was performed for all continuous, nonparametric variables. Data were statistically significant if the probability of a type I error was less than 0.05.
RESULTS
Profiles of cytokines secreted by PBMC from ML and CL patients.
The levels of IFN-γ, TNF-α, IL-10, and IL-5 secreted in vitro by PBMC from ML and CL patients revealed disease-specific patterns (Fig. 1). The mean levels of IFN-γ (3,287 ± 2,442 pg/ml) and TNF-α (1,136 ± 1,023 pg/ml) for the 21 ML patients evaluated were higher than the mean levels of IFN-γ and TNF-α observed in CL patients (1,639 ± 1,245 pg/ml [P = 0.01] and 474 ± 526 pg/ml [P = 0.01], respectively). The IL-5 levels in ML patients were also higher (165 ± 193 pg/ml) than those observed in CL patients (11 ± 18 pg/ml) (P < 0.05). At the same time, the levels of IL-10 in ML patients were significantly lower than those observed in CL patients (20 ± 14 and 59 ± 65 pg/ml, respectively [P < 0.05]).
FIG. 1.
Cytokine profiles in ML and CL patients. IFN-γ and TNF-α production (A) and IL-10 and IL-5 production (B) in PBMC supernatants stimulated with leishmania antigen were determined as described in Materials and Methods. The levels of cytokines were measured by ELISA. Symbols: ▪, ML; ▴, CL.
A comparison of the IFN-γ and TNF-α levels measured before and after therapy for 6 of the 21 ML patients showed that there were unique responses for each cytokine. Whereas there was no significant difference between the levels of IFN-γ before and after antimonial treatment (2,429 ± 653 and 2,091 ± 1,086 pg/ml, respectively [P = 0.5]), the TNF-α levels fell by approximately 60%, from 1,092 ± 442 pg/ml before therapy to 442 ± 296 pg/ml after therapy (P = 0.01).
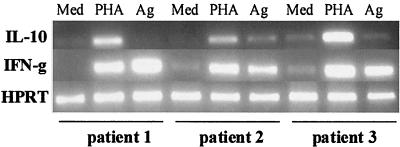
Expression of IL-10 and IFN-γ genes was also evaluated in PBMC cultures of three ML patients by RT-PCR (Fig. 2). All individuals showed similar patterns of strong up-regulation of IFN-γ mRNA after stimulation with leishmania antigen, comparable to the pattern obtained with PHA, as confirmed by densitometry of gels. However, these patterns were not accompanied by corresponding increases in IL-10 gene transcription. Densitometry showed that IL-10 mRNA kept baseline unstimulated levels after exposure to SLA except for patient 2, who was able to produce the transcript, albeit at levels that were less than one-third (29%) of those achieved with mitogen. Furthermore, IL-10 up-regulation following PHA stimulation was similar to up-regulation of IFN-γ, confirming the ability of the PBMC tested to express this cytokine and suggesting that the SLA antigen preparation was a poor inducer of IL-10 in these patients.
FIG. 2.
Cytokine mRNA expression in PBMC from three ML patients with different stimuli. A total of 3 × 106 PBMC were incubated for 72 h in the presence of medium (Med), PHA, or leishmania antigen (Ag), and then total mRNA was extracted and RT-PCR for IL-10 and IFN-γ were performed to assess cytokine gene expression. Hypoxanthine phosphoribosyltransferase (HPRT) expression was used to normalize samples and for comparison.
Cell population responsible for IFN-γ production in ML patients.
To determine the cellular source of IFN-γ synthesis in ML patients, CD4+ T cells, CD8+ T cells, and NK cells were removed from PBMC from six patients (Fig. 3). The IFN-γ levels in supernatants of leishmania antigen-stimulated PBMC were 1,773 ± 777 pg/ml. Removal of CD4+ T cells resulted in lower IFN-γ levels (395 ± 480 pg/ml) (P < 0.005). In contrast, the IFN-γ levels were not affected by removal of CD8+ T cells (1,858 ± 813 pg/ml) and NK cells (1,547 ± 790 pg/ml). Flow cytometric analysis of the PBMC of four ML patients confirmed that CD4+ T cells are responsible for more than 80% of the IFN-γ-producing cells following SLA stimulation (data not shown).
FIG. 3.
Identification of IFN-γ-producing cells in ML patients. As described in Materials and Methods, CD4+ T cells, CD8+ T cells, or NK cells were removed from PBMC by using magnetic beads. Cultures were stimulated with leishmania antigen for 72 h and assayed for the presence of IFN-γ by ELISA. The data are the means and standard deviations for six patients. The percentages of contamination in the populations with cells removed were as follows: population with CD4+ cells removed, 0.2% CD4+ cells; population with CD8+ cells removed, 2% CD8+ cells; and population with NK cells removed, 1% CD56+ cells. An asterisk indicates that the P value is <0.005.
Flow cytometric and cellular removal analyses to determine the sources of TNF-α in ML patients.
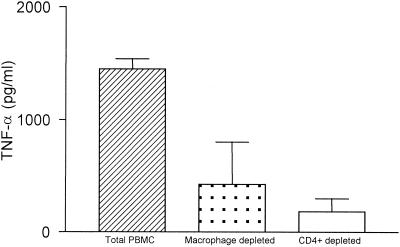
In order to determine the cellular sources of TNF-α in ML patients, macrophages and CD4+ T cells were removed from PBMC from three patients (Fig. 4). The TNF-α levels in the supernatants of PBMC stimulated with leishmania antigen were 1,450 ± 89 pg/ml. Removal of macrophages and CD4+ T cells resulted in lower TNF-α levels (426 ± 376 and 182 ± 117 pg/ml, respectively), suggesting that both of these cell populations are important for production of this cytokine.
FIG. 4.
Identification of TNF-α-producing cells in ML patients. As described in Materials and Methods, CD4+ T cells or macrophages were removed from PBMC, and the PBMC were stimulated with leishmania antigen. TNF-α-producing cells were identified as described in the legend to Fig. 3. The data are the means and standard deviations for three patients. The percentages of contamination in the populations with cells removed were as follows: population with CD4+ cells removed, 0.2% CD4+ cells; and population with CD8+ cells removed, 2% CD8+ cells. The percentages of esterase-positive cells were 28% ± 3% before removal and 2% ± 0.7% after removal.
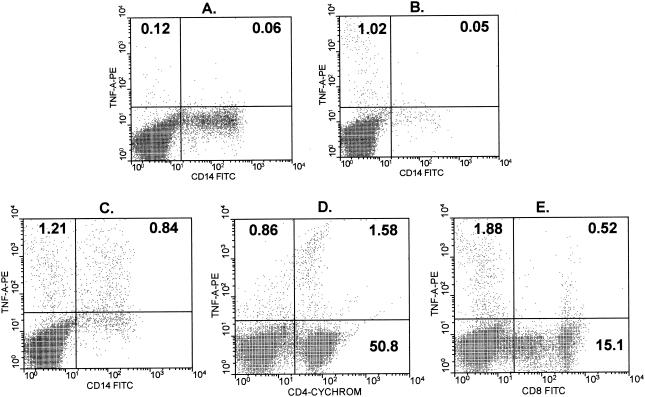
Due to the possibility that removal of macrophages could result in decreased TNF-α production caused by inefficient antigen presentation rather than direct removal of a cytokine-producing cell population, the cellular sources of TNF-α were directly determined with a flow cytometer (Fig. 5). Representative contour plots for two ML patients demonstrate that lymphocytes accounted for the majority (range for the four patients analyzed, 60 to 90%) of the TNF-α-producing cells (Fig. 5B and C), followed by CD14+ monocytes (10 to 40%). The lymphocyte-produced TNF-α came primarily from CD4+ T cells (average, 65%) (Fig. 5D), followed by CD8+ T cells (average, 35%) (Fig. 5E). CD14+ monocytes produced variable amounts of TNF-α in the four patients analyzed by flow cytometry, as shown by the comparison of the profiles for low CD14+ TNF-α expression (Fig. 5B) and high (Fig. 5C) CD14+ TNF-α expression. However, in all cases, the frequency of TNF-α-producing lymphocytes was higher than the frequency of TNF-α-producing CD14+ monocytes, as mentioned above.
FIG. 5.
Cell population responsible for TNF-α production following antigenic stimulation. The cellular sources of TNF-α production are shown for two ML patients, as determined by using single-cell cytoplasmic staining and analysis with a flow cytometer. (A) TNF-α production without stimulus. (B and C) Frequency of CD14+ monocytes which produce TNF-α in two independent individuals, one exhibiting a low frequency of TNF-α-producing monocytes (B) and the other exhibiting a higher frequency of TNF-α-producing monocytes (C) following 20 h of culture with SLA. (D and E) TNF-α-producing CD4+ and CD8+ T cells, respectively, from another ML patient following short-term in vitro stimulation with SLA as described in Materials and Methods. TNF-A-PE, TNF-α-phosphatidylethanolamine; CD4-CYCHROM, CD4-Cychrome.
IL-10 regulation of IFN-γ production in ML patients.
The role of IL-10 in the regulation of IFN-γ production was evaluated by using seven ML patients, seven CL patients, and three healthy subjects. IL-10 was used at concentrations ranging from 0.5 to 20 ng/ml. At low concentrations (0.5 to 2 ng/ml), IL-10 significantly suppressed IFN-γ production in cell cultures prepared from healthy subjects stimulated with PPD, but it had little effect on cell cultures prepared from ML and CL patients stimulated with Leishmania antigen (data not shown). The ability of IL-10 to down-regulate IFN-γ production at a concentration of 5 ng/ml is shown in Fig. 6. The mean IFN-γ levels in cell culture supernatants stimulated with leishmania antigen (10 μg/ml) were 1,641 ± 580, and addition of IL-10 (5 ng/ml) resulted in levels of 1,330 ± 583 pg/ml (19% suppression). These results contrast with those obtained by using PBMC from CL patients. In CL patients, the mean IFN-γ levels in cell culture supernatants stimulated with leishmania antigen were 918 ± 175 pg/ml. Addition of IL-10 at a concentration of 5 ng/ml resulted in IFN-γ levels of 428 ± 24 pg/ml (48% suppression) (P < 0.005). In cell culture supernatants from healthy controls stimulated with PPD (2 μg/ml), the mean IFN-γ levels were 1,529 ± 825 pg/ml, while addition of IL-10 at a concentration of 2 ng/ml resulted in lower IFN-γ levels (214 ±283 pg/ml; 86% suppression). The ability of IL-10 to down-regulate IFN-γ production in ML patient PBMC stimulated with leishmania antigen was tested by using 0.5 to 20 ng of this cytokine per ml. The maximal suppression observed was 22%. In three ML patients, IL-10 (2 ng/ml) down-regulated IFN-γ production by 42% in cultures stimulated with PPD, compared to the 86% down-regulation in the controls (data not shown).
FIG. 6.
Regulation of IFN-γ production by IL-10 in ML and CL patients. As described in Materials and Methods, PBMC from ML or CL patients were stimulated with leishmania antigen (La Ag) in the presence or absence of IL-10. IL-10 was used at a concentration of 5 ng/ml. The data are means and standard deviations for seven ML patients and seven CL patients. The percentage of suppression is indicated next to the single asterisk. Two asterisks indicate that the P value is <0.005.
Modulation of IFN-γ production by TGF-β.
Another cytokine known to inhibit IFN-γ production is TGF-β. Therefore, we also tested the inhibitory effects of TGF-β on IFN-γ production in PBMC from ML patients and CL patients stimulated with leishmania antigen. Addition of TGF-β (20 ng/ml) to PBMC cultures from ML and CL patients resulted in a 15% decrease (from 1,427 ± 434 to 1,210 ± 456 pg/ml) and in no decrease (from 928 ± 204 to 931 ± 202 pg/ml), respectively, in IFN-γ production (P > 0.1), while it suppressed IFN-γ levels up to 47% in cell cultures from healthy subjects stimulated with PPD (from 1,490 ± 896 to 780 ±343 pg/ml).
DISCUSSION
The present study showed that ML is characterized by increased IFN-γ and TNF-α production and decreased secretion of IL-10 in response to leishmania antigens and that the changes are accompanied by a reduced ability of IL-10 and TGF-β to down-regulate these immune responses. IFN-γ, TNF-α, and NO are products that are necessary to kill leishmania (22), but they are also implicated in the inflammation leading to tissue damage (19, 32). Although IFN-γ and TNF-α represent important defense mechanisms against this parasite, previous studies have shown that disease control and cure are not ultimately followed by sterilization (33). This conclusion is supported by the fact that exogenous addition of IFN-γ or of T-cell products from ML patients to human macrophages infected with leishmania does not eliminate the microorganism (12, 28). Thus, overproduction of these proinflammatory cytokines does not necessarily lead to parasite clearance and may be harmful to the host, supporting our hypothesis that ML results from an exacerbated and improperly modulated Th1 immune response to leishmania antigens. In accordance with this reasoning, we have recently shown that the combination of antimony with pentoxifylline, a potent inhibitor of TNF-α production (18), was able to cure ML patients who were refractory to standard antimonial therapy (21). In light of the present results, we suggest that the major contribution of pentoxifylline was to decrease the inflammatory reaction responsible for the tissue damage.
IL-10 is a major cytokine modulating immune responses in leishmaniasis patients. We previously showed that the inability of VL and early CL patients to produce IFN-γ can be restored in vitro by neutralization of IL-10 (10, 30). Conversely, exogenously added IL-10 down-regulates T-cell responses in subjects cured of VL (2). Our demonstration that cells of ML patients have a decreased ability to produce and to respond to IL-10 after stimulation with leishmania antigen suggests that the response to this major immunoregulatory cytokine is impaired in ML patients. This could represent a major mechanism whereby immune responses are deregulated, leading to this severely disfiguring form of human disease.
The pathways underlying the improper production of and response to IL-10 during ML remain elusive. One possibility is that the concentrations of IFN-γ in ML patients down-regulate the expression of IL-10R in lymphocytes and consequently prevent IL-10 modulatory effects. IL-10 acts via a specific ligand (IL-10R) (23), which is structurally related to interferon receptors (20) and might be negatively influenced by high levels of this Th1-type cytokine. Another possibility is that the increased secretion of other proinflammatory cytokines observed in ML patients could also down-regulate IL-10R. In this respect Michel et al. demonstrated that there is decreased expression of the IL-10 receptor in acute exanthematic psoriatic epidermis influenced by the proinflammatory cytokine IL-8 (25).
TGF-β is another cytokine with the ability to modulate immune responses in experimental leishmaniasis, as well as in other conditions (3, 5). For example, TGF-β reduces the antigen-induced cytotoxicity against K562 tumor cells by PBMC of CL patients (4). It has also been reported that addition of TGF-β to PBMC cultures from PPD-skin-test-positive individuals stimulated with PPD results in suppression of IFN-γ production by 70% (26). The data presented here suggest that along with IL-10, TGF-β may play an important regulatory role in ML patients, in which a lack of TGF-β may result in the high damaging levels of IFN-γ and TNF-α observed.
We showed that the major source of leishmania antigen-induced IFN-γ and TNF-α in ML patients is CD4+ Th1 cells. These helper T lymphocytes play a central role in antigen-specific adaptive immune responses, in contrast to the non-antigen-specific innate NK cell response. We propose that improper control of inflammatory reactions mediated by CD4+ cells in ML patients may occur via anergy to and/or lack of adequate levels of IL-10 and TGF-β. A compensatory response leading to down-regulation of the type 1 response might occur through overproduction of Th2-type cytokines, as shown here by the increased IL-5 observed in these patients.
We are tempted to speculate that the biased proinflammatory response described here may be induced in part by selective overexpansion of T-cell clones specific for certain leishmania antigens. Our previous data, as well as the data of other workers, have shown that there are distinct immune responses to recombinant leishmania antigens, as follows: (i) gp63 induces high IFN-γ levels and low IL-4 levels in ML patients (31); (ii) L. braziliensis HSP83 strongly induces mRNA for both Th1-type and Th2-type cytokines in vitro (34); (iii) rLACK generates weak Th1-type responses but apparently activates monocytes (7); and (iv) the elongation initiator factor (LeIF) induces predominantly IL-12 and TNF-α from PBMC of CL and ML patients (35). In keeping with this reasoning, we hypothesize that both host and parasite factors influence the development of ML.
ML is a disease that is associated with a strong Th1-type immune response to leishmania antigen, and parasites are often absent or rarely found in lesions (6). We offer evidence that CD4+ Th1-type cells are responsible for the exaggerated leishmania antigen-specific TNF-α and IFN-γ production in ML patients. This response is poorly regulated by IL-10 and TGF-β. Although Th1-type immunity protects against most forms of leishmaniasis, our data provide evidence that inadequate modulation of a type 1 response may lead to an exaggerated reaction and may constitute the core mechanism driving the immunopathogenesis of ML.
Acknowledgments
This work was supported by an International Research Scholars grant from the Howard Hughes Medical Institute and by National Institutes of Health grant AI-30639. Edgar M. Carvalho is senior investigator of the Brazilian Council of Research (CNPq).
We thank Elbe M. Silva and Jackson L. Moreira for manuscript preparation.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Azulay, R. D., and D. R. Azulay, Jr. 1995. Immune-clinical-pathologic spectrum of leishmaniasis. Int. J. Dermatol. 34:303-307. [DOI] [PubMed] [Google Scholar]
- 2.Bacellar, O., A. D'Oliveira, Jr., S. Jeronimo, and E. M. Carvalho. 2000. IL-10 and IL-12 are the main regulatory cytokines in visceral leishmaniasis. Cytokine 12:1228-1231. [DOI] [PubMed] [Google Scholar]
- 3.Barral, A., M. Barral-Netto, E. C. Yong, C. E. Brownell, D. R. Twardzik, and S. G. Reed. 1993. Transforming growth factor beta as a virulence mechanism for Leishmania braziliensis. Proc. Natl. Acad. Sci. USA 90:3442-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barral-Netto, M., A. Barral, C. Brodskyn, E. M. Carvalho, and S. G. Reed. 1995. Cytotoxicity in human mucosal and cutaneous leishmaniasis. Parasite Immunol. 17:21-28. [DOI] [PubMed] [Google Scholar]
- 5.Barral-Netto, M., A. Barral, C. E. Brownell, Y. A. Skeiky, L. R. Ellingsworth, D. R. Twardzik, and S. G. Reed. 1992. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science 257:545-548. [DOI] [PubMed] [Google Scholar]
- 6.Bittencourt, A. L., and A. Barral. 1991. Evaluation of the histopathological classifications of American cutaneous and mucocutaneous leishmaniasis. Mem. Inst. Oswaldo Cruz Rio De J. 86:51-56. [DOI] [PubMed] [Google Scholar]
- 7.Bottrel, R. L., W. O. Dutra, F. A. Martins, B. Gontijo, E. Carvalho, M. Barral-Netto, A. Barral, R. P. Almeida, W. Mayrink, R. Locksley, and K. J. Gollob. 2001. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble leishmania antigen in human cutaneous leishmaniasis. Infect. Immun. 69:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caceres-Dittmar, G., F. J. Tapia, M. A. Sanchez, M. Yamamura, K. Uyemura, R. L. Modlin, B. R. Bloom, and J. Convit. 1993. Determination of the cytokine profile in American cutaneous leishmaniasis using the polymerase chain reaction. Clin. Exp. Immunol. 91:500-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho, E. M., O. Bacellar, A. Barral, R. Badaro, and W. D. Johnson, Jr. 1989. Antigen-specific immunosuppression in visceral leishmaniasis is cell mediated. J. Clin. Investig. 83:860-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho, E. M., O. Bacellar, C. Brownell, T. Regis, R. L. Coffman, and S. G. Reed. 1994. Restoration of IFN-gamma production and lymphocyte proliferation in visceral leishmaniasis. J. Immunol. 152:5949-5956. [PubMed] [Google Scholar]
- 11.Carvalho, E. M., A. Barral, D. Pedral-Sampaio, M. Barral-Netto, R. Badaro, H. Rocha, and W. D. Johnson, Jr. 1992. Immunologic markers of clinical evolution in children recently infected with Leishmania donovani chagasi. J. Infect. Dis. 165:535-540. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho, E. M., W. D. Johnson, E. Barreto, P. D. Marsden, J. L. Costa, S. Reed, and H. Rocha. 1985. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J. Immunol. 135:4144-4148. [PubMed] [Google Scholar]
- 13.Castes, M., A. Agnelli, and A. J. Rondon. 1984. Mechanisms associated with immunoregulation in human American cutaneous leishmaniasis. Clin. Exp. Immunol. 57:279-286. [PMC free article] [PubMed] [Google Scholar]
- 14.Castes, M., A. Agnelli, O. Verde, and A. J. Rondon. 1983. Characterization of the cellular immune response in American cutaneous leishmaniasis. Clin. Immunol. Immunopathol. 27:176-186. [DOI] [PubMed] [Google Scholar]
- 15.Castes, M., D. Trujillo, M. E. Rojas, C. T. Fernandez, L. Araya, M. Cabrera, J. Blackwell, and J. Convit. 1993. Serum levels of tumor necrosis factor in patients with American cutaneous leishmaniasis. Biol. Res. 26:233-238. [PubMed] [Google Scholar]
- 16.Cuba Cuba, C. A., P. D. Marsden, A. C. Barreto, I. Roitman, A. Vexenat, L. M. de Lima, and M. H. de Sa. 1984. Identification of human stocks of Leishmania spp. isolated from patients with mucocutaneous leishmaniasis in Tres Bracos, Bahia, Brazil. Trans. R. Soc. Trop. Med. Hyg. 78:708-710. [DOI] [PubMed] [Google Scholar]
- 17.Da Cruz, A. M., M. P. de Oliveira, P. M. De Luca, S. C. Mendonca, and S. G. Coutinho. 1996. Tumor necrosis factor-alpha in human American tegumentary leishmaniasis. Mem. Inst. Oswaldo Cruz Rio De J. 91:225-229. [DOI] [PubMed] [Google Scholar]
- 18.Doherty, G. M., J. C. Jensen, H. R. Alexander, C. M. Buresh, and J. A. Norton. 1991. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery 110:192-198. [PubMed] [Google Scholar]
- 19.Grau, G. E., P. F. Piguet, P. Vassalli, and P. H. Lambert. 1989. Tumor-necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol. Rev. 112:49-70. [DOI] [PubMed] [Google Scholar]
- 20.Ho, A. S., Y. Liu, T. A. Khan, D. H. Hsu, J. F. Bazan, and K. W. Moore. 1993. A receptor for interleukin 10 is related to interferon receptors. Proc. Natl. Acad. Sci. USA 90:11267-11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lessa, H. A., P. Machado, F. Lima, A. A. Cruz, O. Bacellar, J. Guerreiro, and E. M. Carvalho. 2001. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. Am. J. Trop. Med. Hyg. 65:87-89. [DOI] [PubMed] [Google Scholar]
- 22.Liew, F. Y., Y. Li, and S. Millott. 1990. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J. Immunol. 145:4306-4310. [PubMed] [Google Scholar]
- 23.Liu, Y., S. H. Wei, A. S. Ho, R. de Waal Malefyt, and K. W. Moore. 1994. Expression cloning and characterization of a human IL-10 receptor. J. Immunol. 152:1821-1829. [PubMed] [Google Scholar]
- 24.Marsden, P. D., and T. C. Jones. 1985. Clinical manifestations, diagnosis and treatment of leishmaniasis. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 25.Michel, G., A. Mirmohammadsadegh, E. Olasz, B. Jarzebska-Deussen, A. Muschen, L. Kemeny, H. F. Abts, and T. Ruzicka. 1997. Demonstration and functional analysis of IL-10 receptors in human epidermal cells: decreased expression in psoriatic skin, down-modulation by IL-8, and up-regulation by an antipsoriatic glucocorticosteroid in normal cultured keratinocytes. J. Immunol. 159:6291-6297. [PubMed] [Google Scholar]
- 26.Othieno, C., C. S. Hirsch, B. D. Hamilton, K. Wilkinson, J. J. Ellner, and Z. Toossi. 1999. Interaction of Mycobacterium tuberculosis-induced transforming growth factor β1 and interleukin-10. Infect. Immun. 67:5730-5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pirmez, C., M. Yamamura, K. Uyemura, M. Paes-Oliveira, F. Conceicao-Silva, and R. L. Modlin. 1993. Cytokine patterns in the pathogenesis of human leishmaniasis. J. Clin. Investig. 91:1390-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed, S. G., J. S. da Silva, J. L. Ho, J. K. Koehler, D. M. Russo, D. L. Pihl, and R. W. Coombs. 1992. Cytokine activation of human macrophages infected with HIV-1 to inhibit intracellular protozoa. J. Acquir. Immune Defic. Syndr. 5:666-675. [PubMed] [Google Scholar]
- 29.Ribeiro-de-Jesus, A., R. P. Almeida, H. Lessa, O. Bacellar, and E. M. Carvalho. 1998. Cytokine profile and pathology in human leishmaniasis. Braz. J. Med. Biol. Res. 31:143-148. [DOI] [PubMed] [Google Scholar]
- 30.Rocha, P. N., R. P. Almeida, O. Bacellar, A. R. de Jesus, D. C. Filho, A. C. Filho, A. Barral, R. L. Coffman, and E. M. Carvalho. 1999. Down-regulation of Th1 type of response in early human American cutaneous leishmaniasis. J. Infect. Dis. 180:1731-1734. [DOI] [PubMed] [Google Scholar]
- 31.Russo, D. M., M. Barral-Netto, A. Barral, and S. G. Reed. 1993. Human T-cell responses in leishmania infections. Prog. Clin. Parasitol. 3:119-144. [DOI] [PubMed] [Google Scholar]
- 32.Sarno, E. N., G. E. Grau, L. M. Vieira, and J. A. Nery. 1991. Serum levels of tumour necrosis factor-alpha and interleukin-1 beta during leprosy reactional states. Clin. Exp. Immunol. 84:103-108. [PMC free article] [PubMed] [Google Scholar]
- 33.Schubach, A., M. C. Marzochi, T. Cuzzi-Maya, A. V. Oliveira, M. L. Araujo, A. L. Oliveira, R. S. Pacheco, H. Momen, F. Conceicao-Silva, S. G. Coutinho, and K. B. Marzochi. 1998. Cutaneous scars in American tegumentary leishmaniasis patients: a site of Leishmania (Viannia) braziliensis persistence and viability eleven years after antimonial therapy and clinical cure. Am. J. Trop. Med. Hyg. 58:824-827. [DOI] [PubMed] [Google Scholar]
- 34.Skeiky, Y. A., D. R. Benson, J. A. Guderian, J. A. Whittle, O. Bacelar, E. M. Carvalho, and S. G. Reed. 1995. Immune responses of leishmaniasis patients to heat shock proteins of Leishmania species and humans. Infect. Immun. 63:4105-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skeiky, Y. A., J. A. Guderian, D. R. Benson, O. Bacelar, E. M. Carvalho, M. Kubin, R. Badaro, G. Trinchieri, and S. G. Reed. 1995. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce interleukin 12. J. Exp. Med. 181:1527-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sornasse, T., P. V. Larenas, K. A. Davis, J. E. de Vries, and H. Yssel. 1996. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+ T cells, analyzed at the single-cell level. J. Exp. Med. 184:473-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein, M., and S. Gordon. 1991. Regulation of tumor necrosis factor (TNF) release by murine peritoneal macrophages: role of cell stimulation and specific phagocytic plasma membrane receptors. Eur. J. Immunol. 21:431-437. [DOI] [PubMed] [Google Scholar]