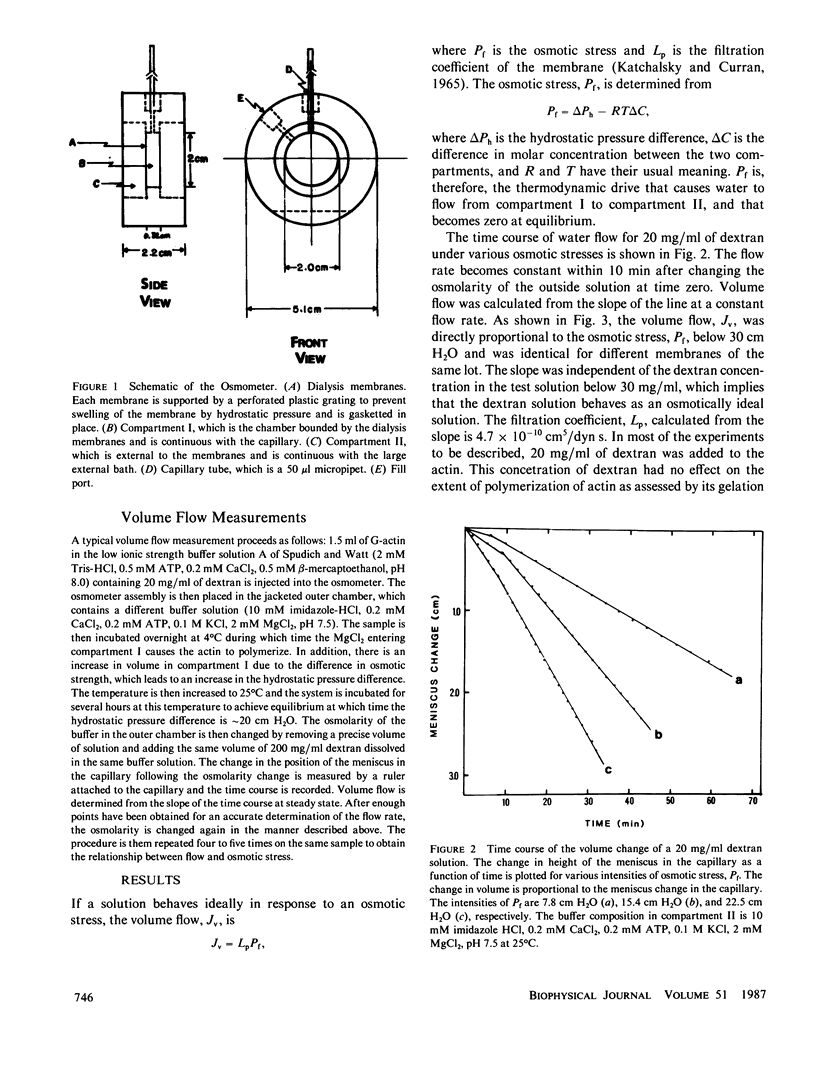
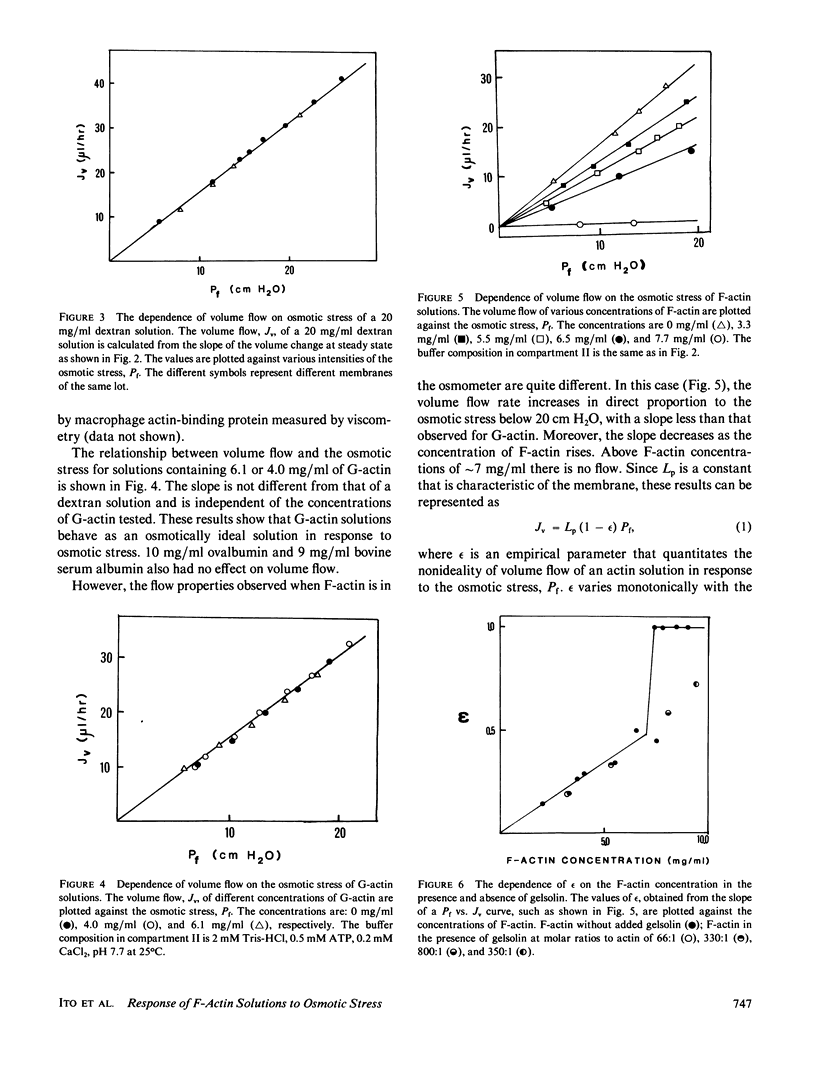
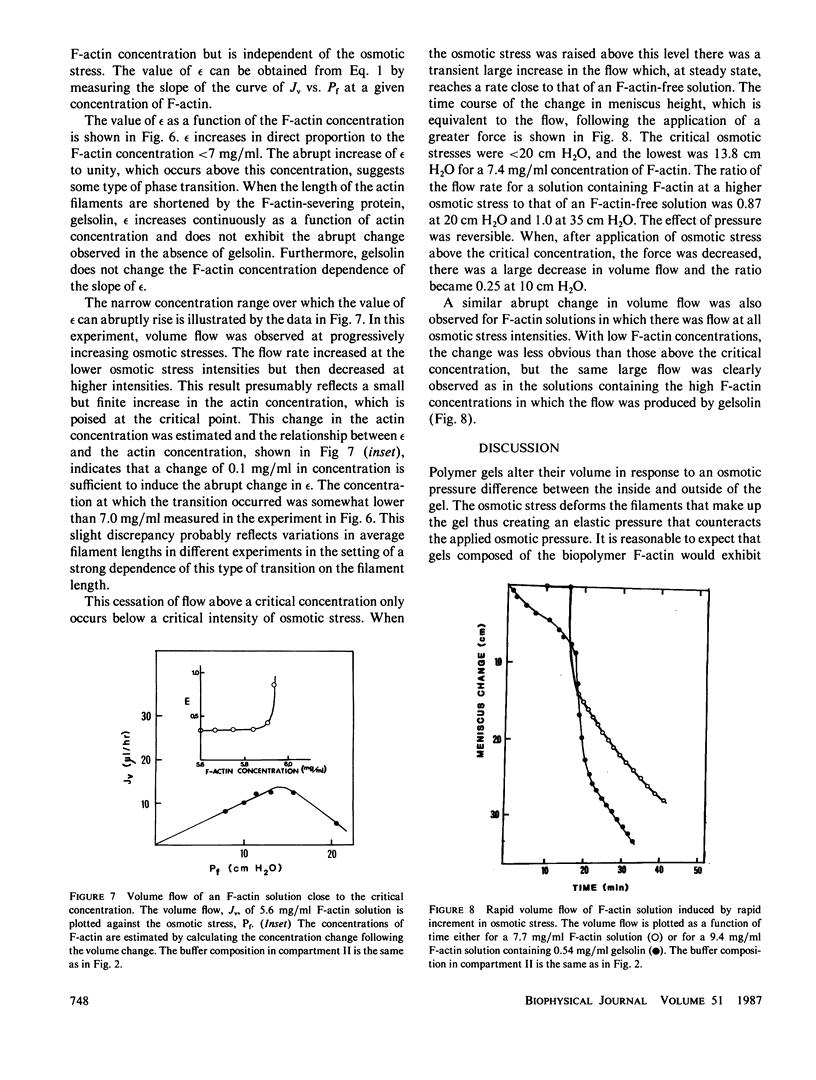
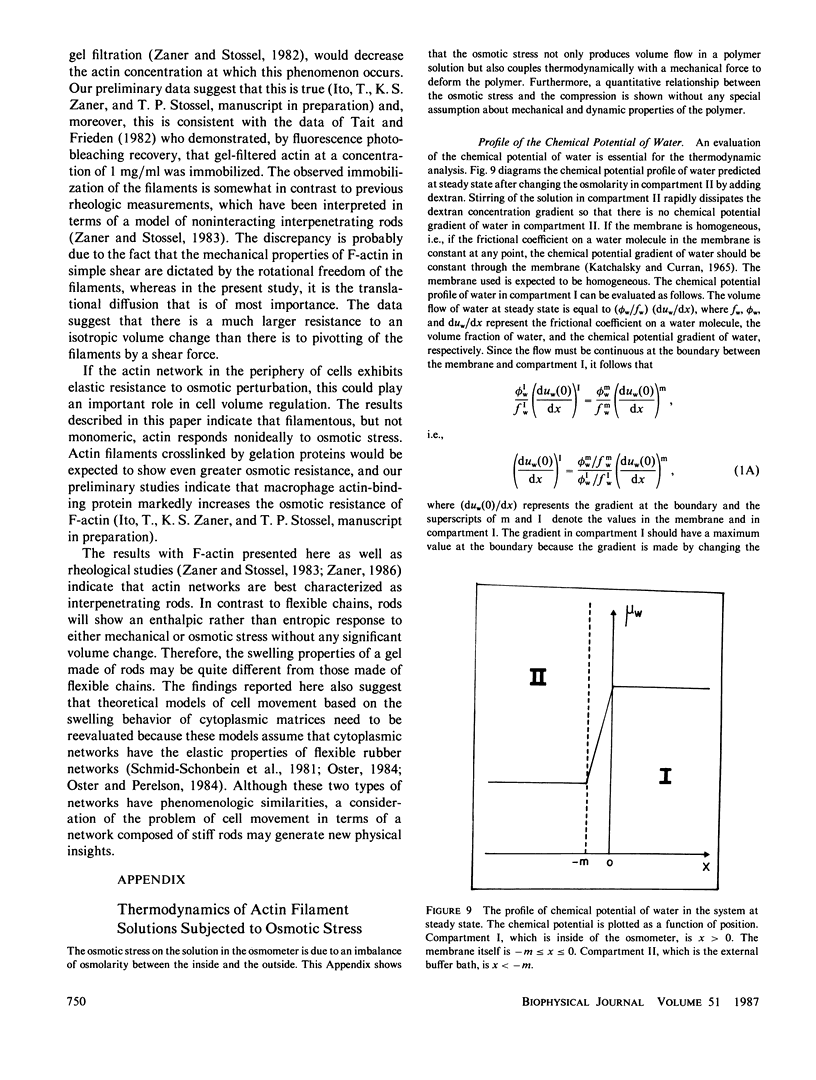
Abstract
Ovalbumin and G-actin solutions decreased their volume in a concentration-dependent manner in response to an osmotic stress, arising from an osmotic pressure gradient of 5-20 cm H2O at 25 degrees C, at protein concentrations as high as 20 mg/ml. In contrast, solutions of F-actin exhibited a concentration-dependent decrease in their rate of volume change in response to the osmotic stress. Shortening of F-actin by gelsolin did not affect this decrease, suggesting that the elastic response of the filaments underlies the osmotically nonideal behavior. However, above a critical actin concentration of approximately 7 mg/ml, no volume change occurred in response to osmotic gradients as high as 20 cm H2O. The concentration at which this critical phenomenon occurred and its abolition by shortening of F-actin by gelsolin suggest that a transition of diffusible rods to a glassy state is the cause of this critical phenomenon. Above the critical concentration, an increase in the osmotic pressure applied to an F-actin solution to greater than 20 cm H2O produced a transient increase in flow rate to that expected for a solution containing no polymer. This finding may represent a transition from an isotropic glassy state to an anisotropic and heterogeneous one wherein regions of pure solvent coexist with domains of pure polymer.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaponnier C., Janmey P. A., Yin H. L. The actin filament-severing domain of plasma gelsolin. J Cell Biol. 1986 Oct;103(4):1473–1481. doi: 10.1083/jcb.103.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster G. F. On the crawling of cells. J Embryol Exp Morphol. 1984 Nov;83 (Suppl):329–364. [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Sung K. L., Tözeren H., Skalak R., Chien S. Passive mechanical properties of human leukocytes. Biophys J. 1981 Oct;36(1):243–256. doi: 10.1016/S0006-3495(81)84726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Tait J. F., Frieden C. Polymerization and gelation of actin studied by fluorescence photobleaching recovery. Biochemistry. 1982 Jul 20;21(15):3666–3674. doi: 10.1021/bi00258a022. [DOI] [PubMed] [Google Scholar]
- Tanaka T. Gels. Sci Am. 1981 Jan;244(1):124-36, 138. doi: 10.1038/scientificamerican0181-124. [DOI] [PubMed] [Google Scholar]
- Zaner K. S., Stossel T. P. Physical basis of the rheologic properties of F-actin. J Biol Chem. 1983 Sep 25;258(18):11004–11009. [PubMed] [Google Scholar]
- Zaner K. S., Stossel T. P. Some perspectives on the viscosity of actin filaments. J Cell Biol. 1982 Jun;93(3):987–991. doi: 10.1083/jcb.93.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaner K. S. The effect of the 540-kilodalton actin cross-linking protein, actin-binding protein, on the mechanical properties of F-actin. J Biol Chem. 1986 Jun 15;261(17):7615–7620. [PubMed] [Google Scholar]


