Abstract
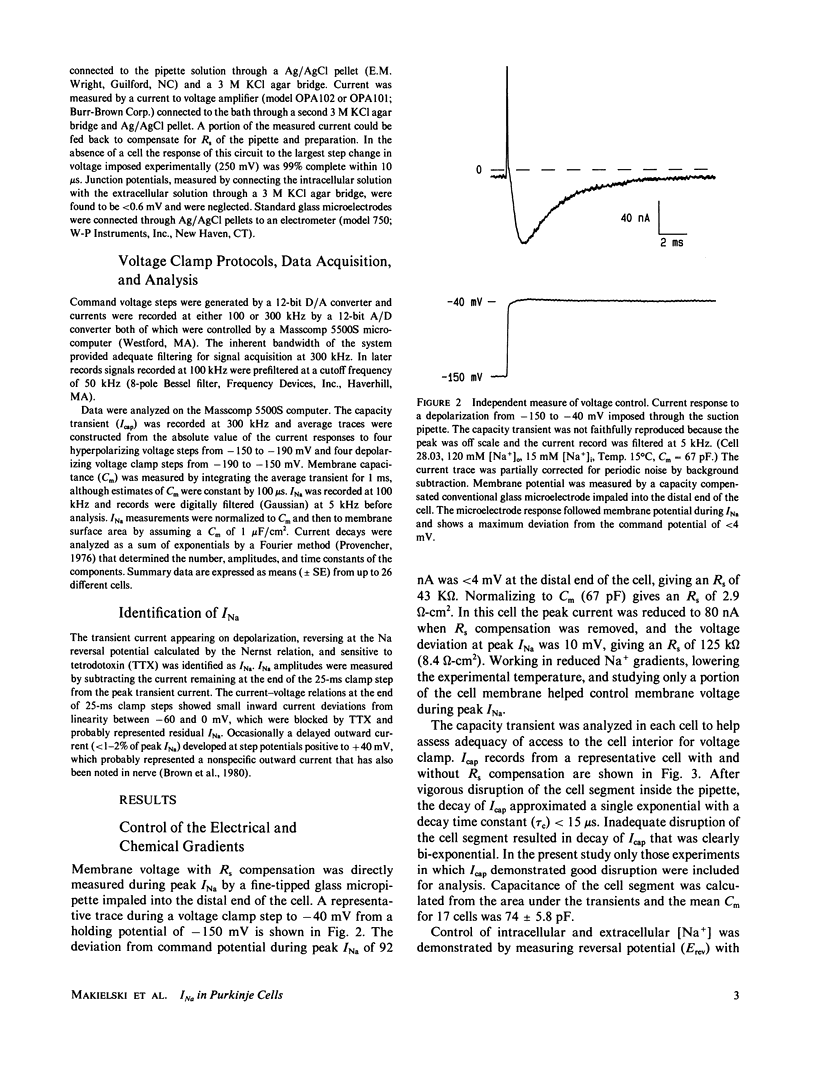
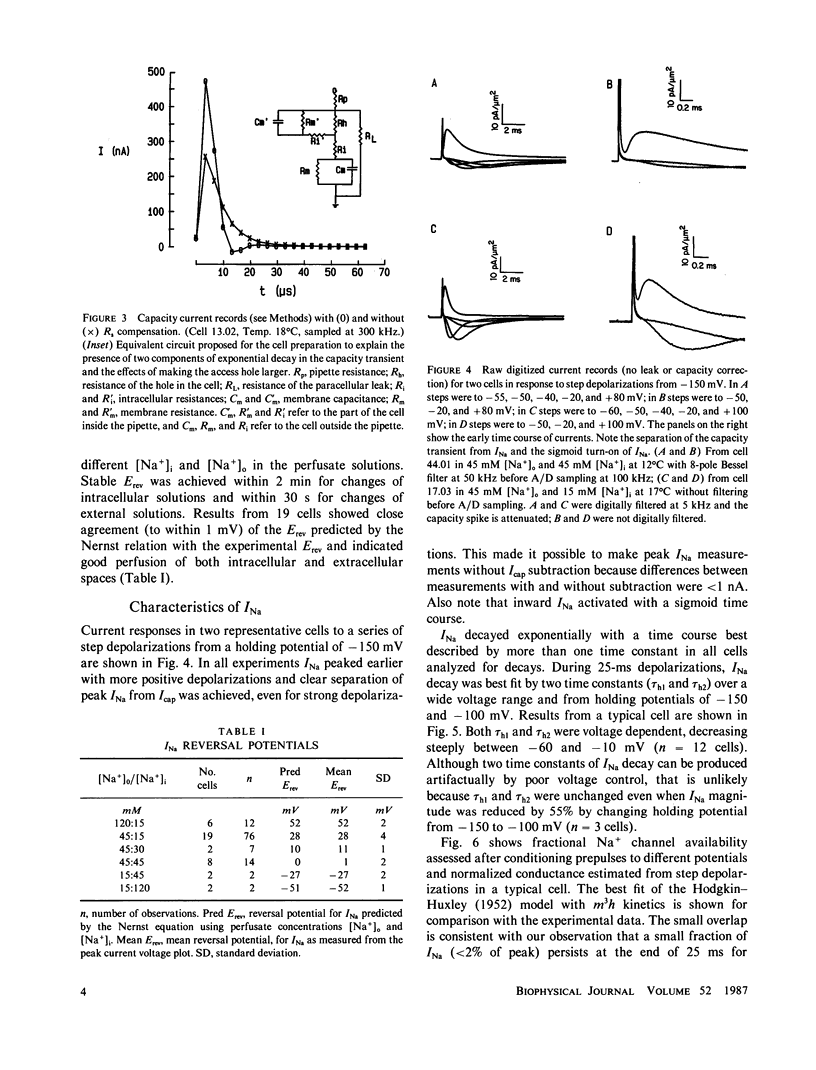
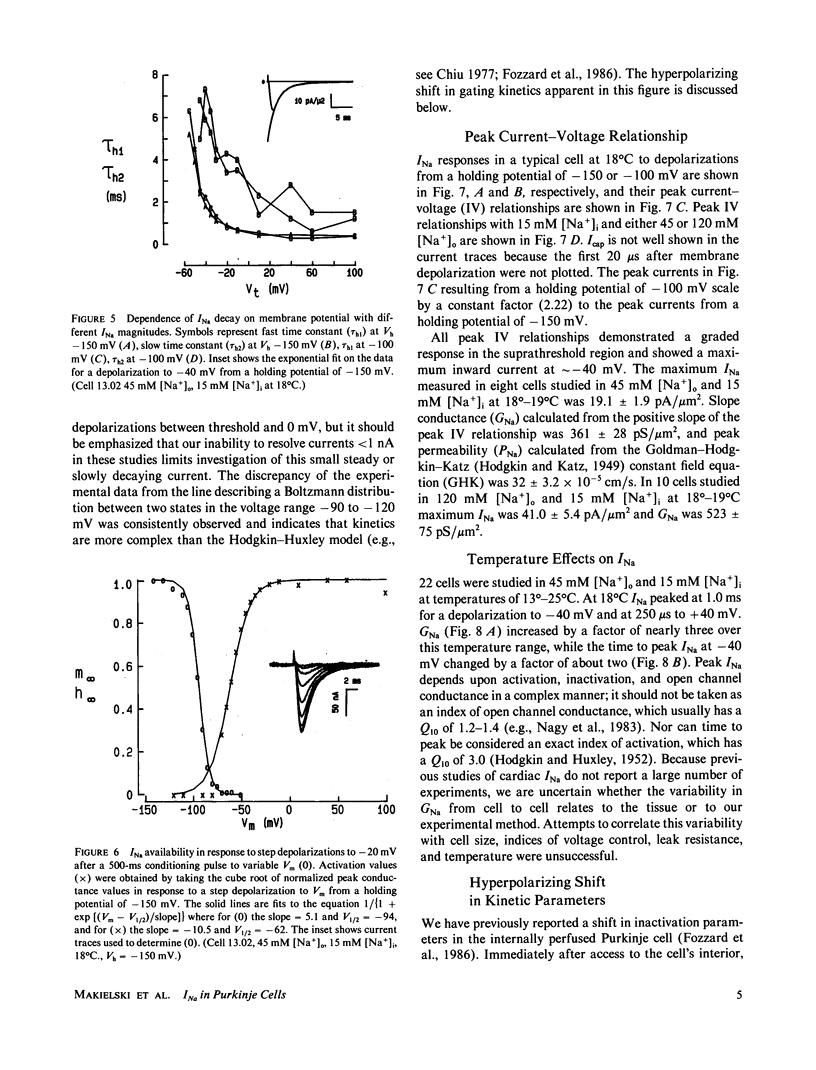
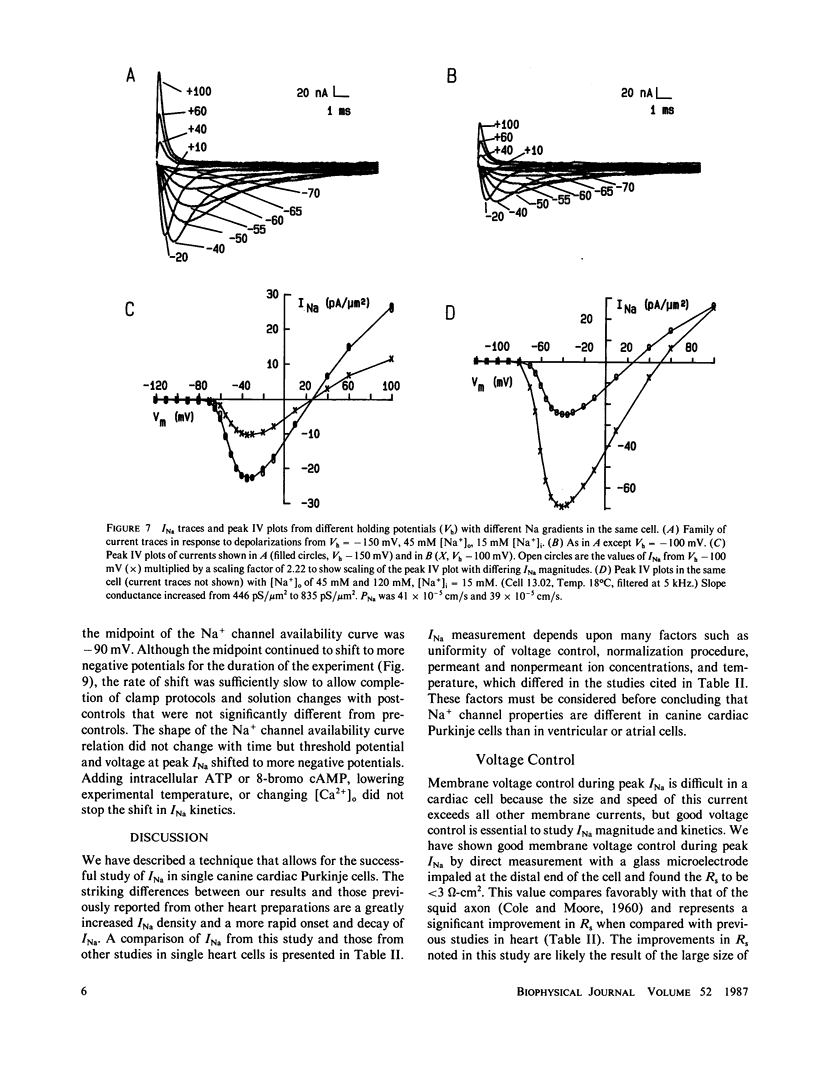
Study of the excitatory sodium current (INa) intact heart muscle has been hampered by the limitations of voltage clamp methods in multicellular preparations that result from the presence of large series resistance and from extracellular ion accumulation and depletion. To minimize these problems we voltage clamped and internally perfused freshly isolated canine cardiac Purkinje cells using a large bore (25-microns diam) double-barreled flow-through glass suction pipette. Control of [Na+]i was demonstrated by the agreement of measured INa reversal potentials with the predictions of the Nernst relation. Series resistance measured by an independent microelectrode was comparable to values obtained in voltage clamp studies of squid axons (less than 3.0 omega-cm2). The rapid capacity transient decays (tau c less than 15 microseconds) and small deviations of membrane potential (less than 4 mV at peak INa) achieved in these experiments represent good conditions for the study of INa. We studied INa in 26 cells (temperature range 13 degrees-24 degrees C) with 120 or 45 mM [Na+]o and 15 mM [Na+]i. Time to peak INa at 18 degrees C ranged from 1.0 ms (-40 mV) to less than 250 microseconds (+ 40 mV), and INa decayed with a time course best described by two time constants in the voltage range -60 to -10 mV. Normalized peak INa in eight cells at 18 degrees C was 2.0 +/- 0.2 mA/cm2 with [Na+]o 45 mM and 4.1 +/- 0.6 mA/cm2 with [Na+]o 120 mM. These large peak current measurements require a high density of Na+ channels. It is estimated that 67 +/- 6 channels/micron 2 are open at peak INa, and from integrated INa as many as 260 Na+ channels/micron2 are available for opening in canine cardiac Purkinje cells.
Full text
PDF

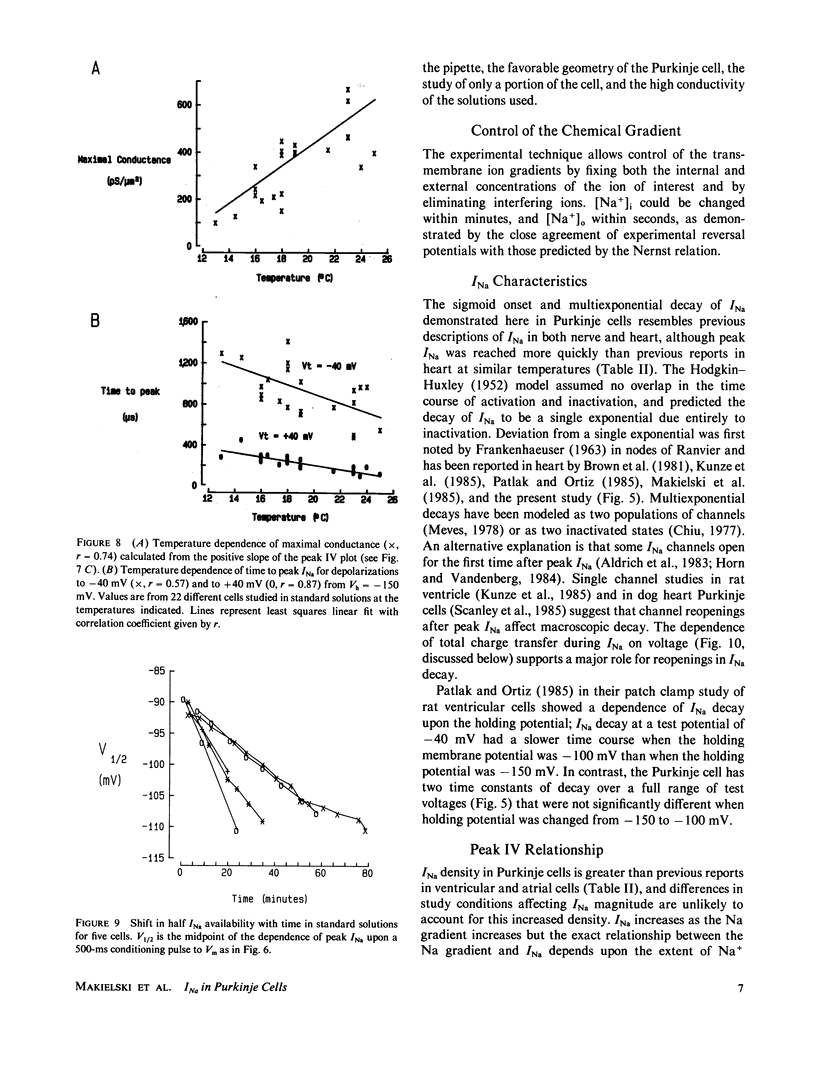
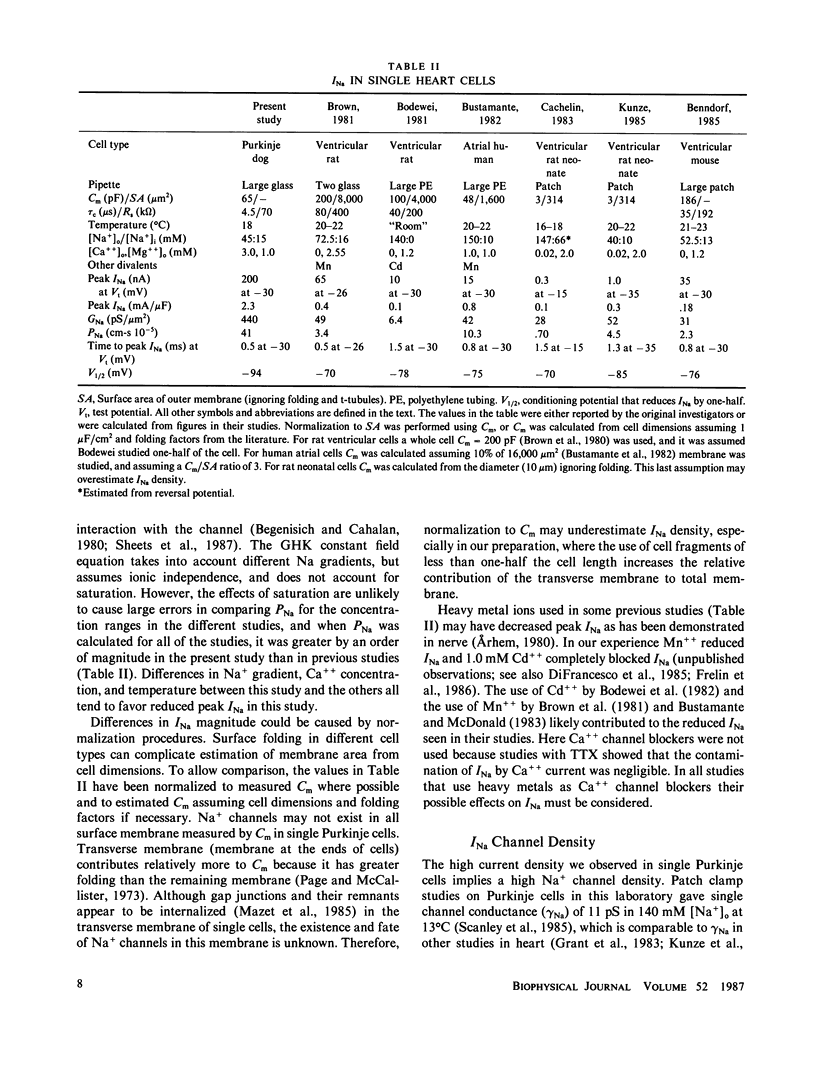
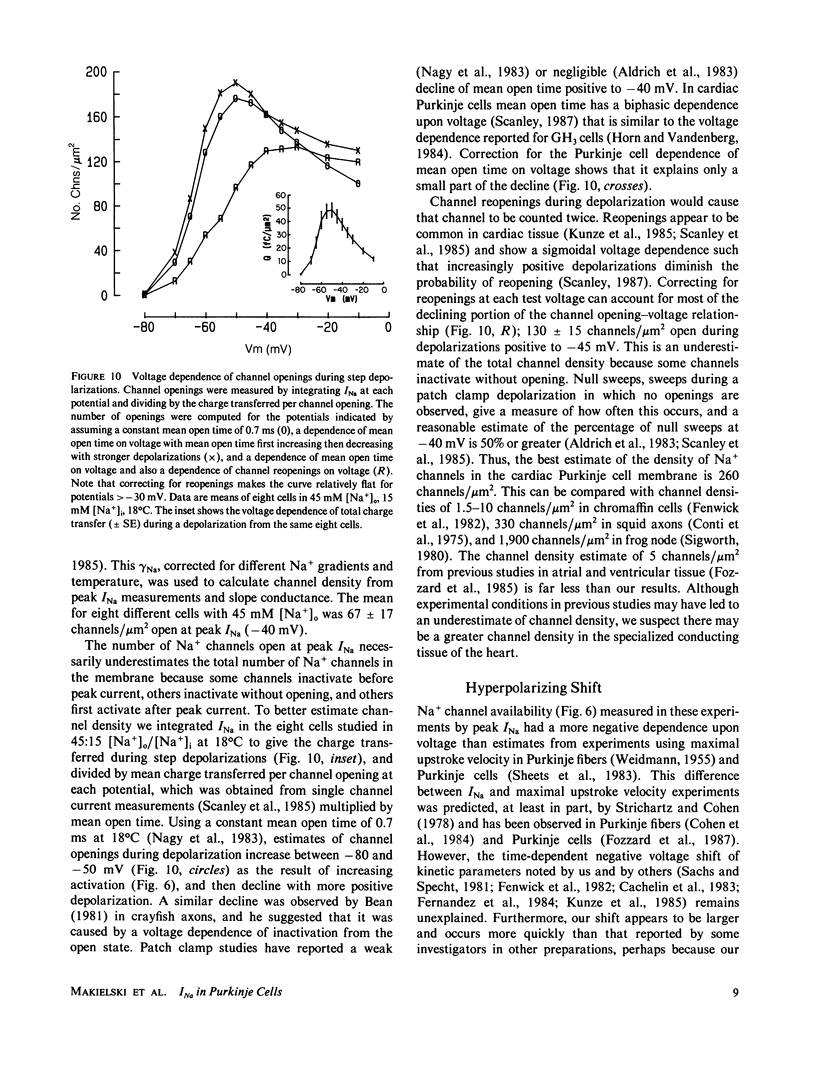


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Arhem P. Effects of some heavy metal ions on the ionic currents of myelinated fibres from Xenopus laevis. J Physiol. 1980 Sep;306:219–231. doi: 10.1113/jphysiol.1980.sp013393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Sodium channel inactivation in the crayfish giant axon. Must channels open before inactivating? Biophys J. 1981 Sep;35(3):595–614. doi: 10.1016/S0006-3495(81)84815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., McGuigan J. A. Voltage clamping of multicellular myocardial preparations: capabilities and limitations of existing methods. Prog Biophys Mol Biol. 1978;34(3):219–254. doi: 10.1016/0079-6107(79)90019-1. [DOI] [PubMed] [Google Scholar]
- Begenisich T. B., Cahalan M. D. Sodium channel permeation in squid axons. II: Non-independence and current-voltage relations. J Physiol. 1980 Oct;307:243–257. doi: 10.1113/jphysiol.1980.sp013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benndorf K., Boldt W., Nilius B. Sodium current in single myocardial mouse cells. Pflugers Arch. 1985 May;404(2):190–196. doi: 10.1007/BF00585418. [DOI] [PubMed] [Google Scholar]
- Bodewei R., Hering S., Lemke B., Rosenshtraukh L. V., Undrovinas A. I., Wollenberger A. Characterization of the fast sodium current in isolated rat myocardial cells: simulation of the clamped membrane potential. J Physiol. 1982 Apr;325:301–315. doi: 10.1113/jphysiol.1982.sp014151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981 Sep;318:479–500. doi: 10.1113/jphysiol.1981.sp013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Morimoto K., Tsuda Y., wilson D. L. Calcium current-dependent and voltage-dependent inactivation of calcium channels in Helix aspersa. J Physiol. 1981 Nov;320:193–218. doi: 10.1113/jphysiol.1981.sp013944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J. O., McDonald T. F. Sodium currents in segments of human heart cells. Science. 1983 Apr 15;220(4594):320–321. doi: 10.1126/science.6301004. [DOI] [PubMed] [Google Scholar]
- Bustamante J. O., Watanabe T., Murphy D. A., McDonald T. F. Isolation of single atrial and ventricular cells from the human heart. Can Med Assoc J. 1982 Apr 1;126(7):791–793. [PMC free article] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Ionic current measurements in the squid giant axon membrane. J Gen Physiol. 1960 Sep;44:123–167. doi: 10.1085/jgp.44.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachelin A. B., De Peyer J. E., Kokubun S., Reuter H. Sodium channels in cultured cardiac cells. J Physiol. 1983 Jul;340:389–401. doi: 10.1113/jphysiol.1983.sp014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. J., Bean B. P., Tsien R. W. Maximal upstroke velocity as an index of available sodium conductance. Comparison of maximal upstroke velocity and voltage clamp measurements of sodium current in rabbit Purkinje fibers. Circ Res. 1984 Jun;54(6):636–651. doi: 10.1161/01.res.54.6.636. [DOI] [PubMed] [Google Scholar]
- Colatsky T. J. Voltage clamp measurements of sodium channel properties in rabbit cardiac Purkinje fibres. J Physiol. 1980 Aug;305:215–234. doi: 10.1113/jphysiol.1980.sp013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., De Felice L. J., Wanke E. Potassium and sodium ion current noise in the membrane of the squid giant axon. J Physiol. 1975 Jun;248(1):45–82. doi: 10.1113/jphysiol.1975.sp010962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Shigeto N., Lieberman M., Johnson E. A. The initial inward current in spherical clusters of chick embryonic heart cells. J Gen Physiol. 1980 Apr;75(4):437–456. doi: 10.1085/jgp.75.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. INACTIVATION OF THE SODIUM-CARRYING MECHANISM IN MYELINATED NERVE FIBRES OF XENOPUS LAEVIS. J Physiol. 1963 Nov;169:445–451. doi: 10.1113/jphysiol.1963.sp007271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Fox A. P., Krasne S. Membrane patches and whole-cell membranes: a comparison of electrical properties in rat clonal pituitary (GH3) cells. J Physiol. 1984 Nov;356:565–585. doi: 10.1113/jphysiol.1984.sp015483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard H. A., Beeler G. W., Jr The voltage clamp and cardiac electrophysiology. Circ Res. 1975 Oct;37(4):403–413. doi: 10.1161/01.res.37.4.403. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., January C. T., Makielski J. C. New studies of the excitatory sodium currents in heart muscle. Circ Res. 1985 Apr;56(4):475–485. doi: 10.1161/01.res.56.4.475. [DOI] [PubMed] [Google Scholar]
- Frelin C., Cognard C., Vigne P., Lazdunski M. Tetrodotoxin-sensitive and tetrodotoxin-resistant Na+ channels differ in their sensitivity to Cd2+ and Zn2+. Eur J Pharmacol. 1986 Mar 18;122(2):245–250. doi: 10.1016/0014-2999(86)90109-3. [DOI] [PubMed] [Google Scholar]
- Grant A. O., Starmer C. F., Strauss H. C. Unitary sodium channels in isolated cardiac myocytes of rabbit. Circ Res. 1983 Dec;53(6):823–829. doi: 10.1161/01.res.53.6.823. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A. Statistical properties of single sodium channels. J Gen Physiol. 1984 Oct;84(4):505–534. doi: 10.1085/jgp.84.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G. Intracellular perfusion of nerve cells and its effects on membrane currents. Physiol Rev. 1984 Apr;64(2):435–454. doi: 10.1152/physrev.1984.64.2.435. [DOI] [PubMed] [Google Scholar]
- Kunze D. L., Lacerda A. E., Wilson D. L., Brown A. M. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol. 1985 Nov;86(5):691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze D. L., Lacerda A. E., Wilson D. L., Brown A. M. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol. 1985 Nov;86(5):691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazet F., Wittenberg B. A., Spray D. C. Fate of intercellular junctions in isolated adult rat cardiac cells. Circ Res. 1985 Feb;56(2):195–204. doi: 10.1161/01.res.56.2.195. [DOI] [PubMed] [Google Scholar]
- Meves H. Inactivation of the sodium permeability in squid giant nerve fibres. Prog Biophys Mol Biol. 1978;33(2):207–230. doi: 10.1016/0079-6107(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Nagy K., Kiss T., Hof D. Single Na channels in mouse neuroblastoma cell membrane. Indications for two open states. Pflugers Arch. 1983 Dec;399(4):302–308. doi: 10.1007/BF00652757. [DOI] [PubMed] [Google Scholar]
- Page E., McCallister L. P. Studies on the intercalated disk of rat left ventricular myocardial cells. J Ultrastruct Res. 1973 Jun;43(5):388–411. doi: 10.1016/s0022-5320(73)90017-8. [DOI] [PubMed] [Google Scholar]
- Provencher S. W. A Fourier method for the analysis of exponential decay curves. Biophys J. 1976 Jan;16(1):27–41. doi: 10.1016/S0006-3495(76)85660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogart R. Sodium channels in nerve and muscle membrane. Annu Rev Physiol. 1981;43:711–725. doi: 10.1146/annurev.ph.43.030181.003431. [DOI] [PubMed] [Google Scholar]
- Sheets M. F., January C. T., Fozzard H. A. Isolation and characterization of single canine cardiac purkinje cells. Circ Res. 1983 Oct;53(4):544–548. doi: 10.1161/01.res.53.4.544. [DOI] [PubMed] [Google Scholar]
- Sheets M. F., Scanley B. E., Hanck D. A., Makielski J. C., Fozzard H. A. Open sodium channel properties of single canine cardiac Purkinje cells. Biophys J. 1987 Jul;52(1):13–22. doi: 10.1016/S0006-3495(87)83183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. The conductance of sodium channels under conditions of reduced current at the node of Ranvier. J Physiol. 1980 Oct;307:131–142. doi: 10.1113/jphysiol.1980.sp013427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G., Cohen I. Vmax as a measure of GNa in nerve and cardiac membranes. Biophys J. 1978 Jul;23(1):153–156. doi: 10.1016/S0006-3495(78)85440-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. The effect of the cardiac membrane potential on the rapid availability of the sodium-carrying system. J Physiol. 1955 Jan 28;127(1):213–224. doi: 10.1113/jphysiol.1955.sp005250. [DOI] [PMC free article] [PubMed] [Google Scholar]



