Abstract
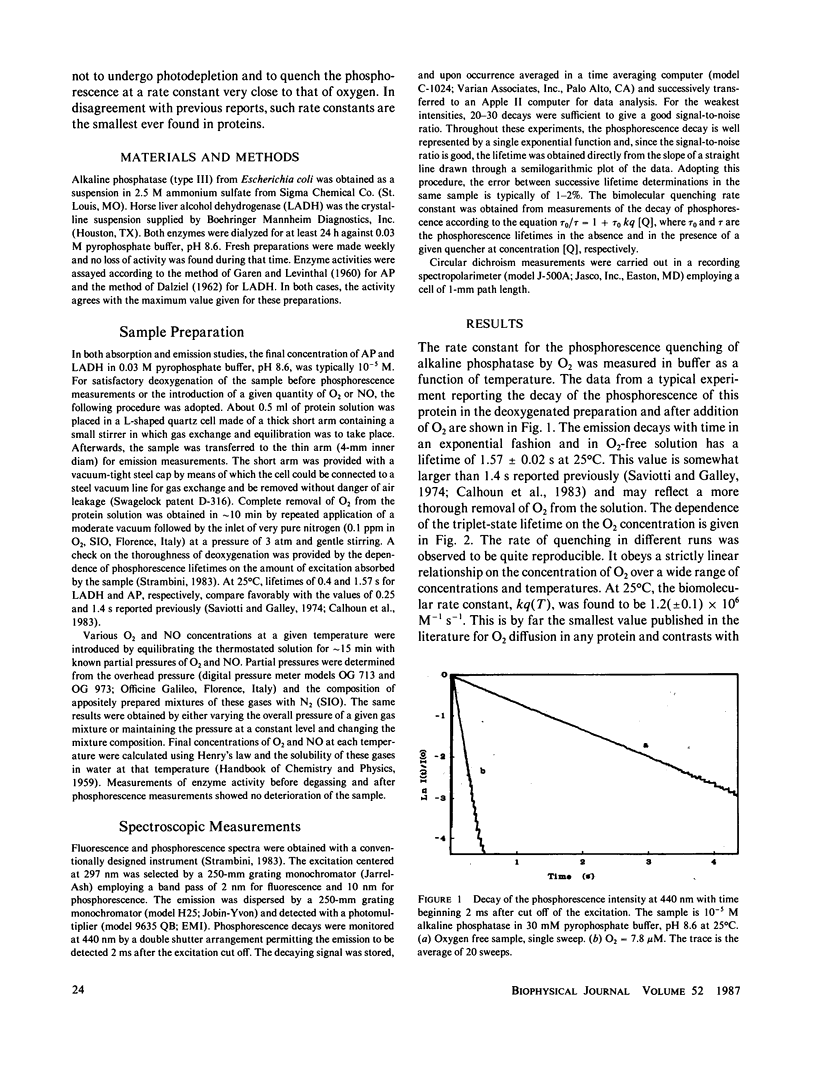
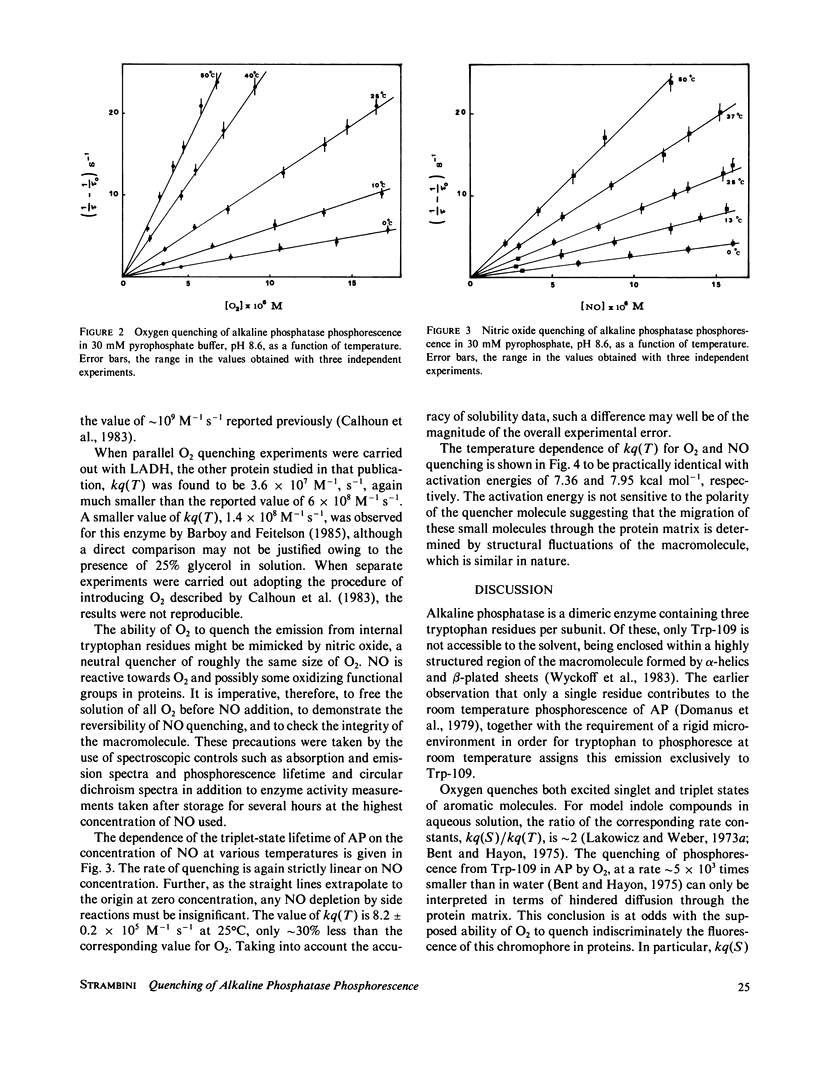
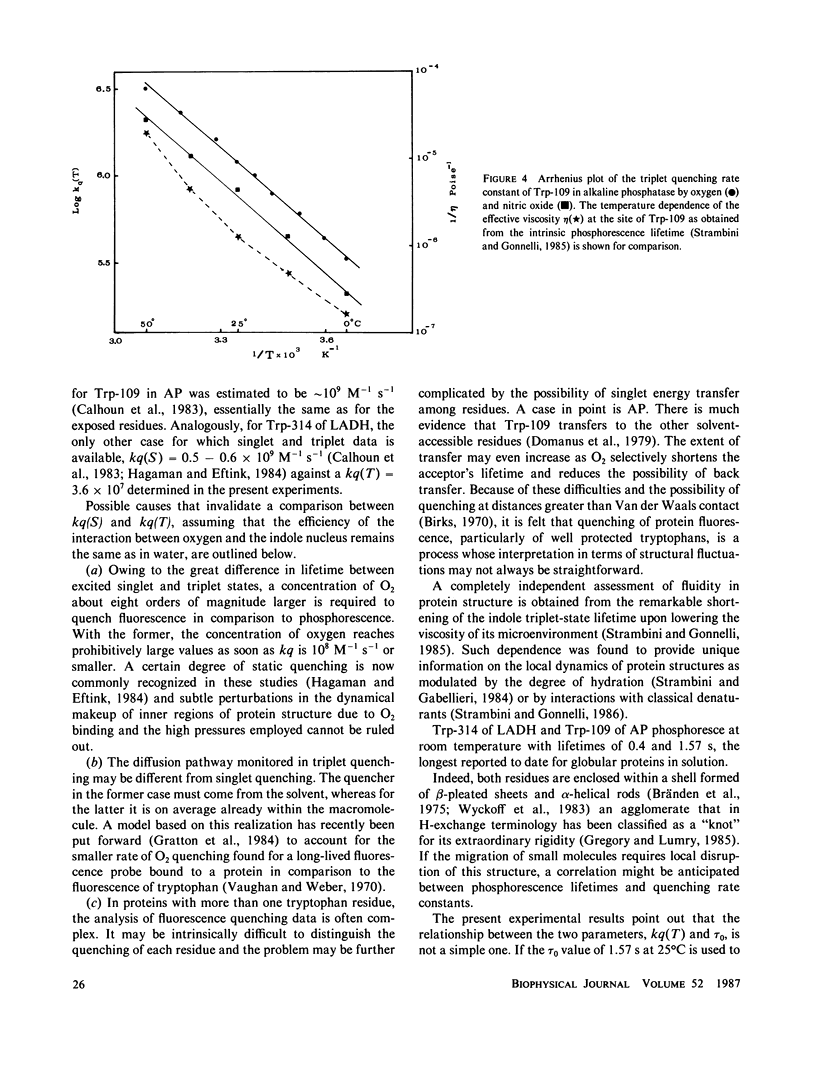
The rate constant for quenching the phosphorescence of alkaline phosphatase by molecular oxygen was measured as a function of temperature. The results disagree with previous determinations and, contrary to fluorescence quenching, show that diffusion of O2 to this region of the macromolecule is a highly hindered process. When nitric oxide is introduced as a quencher, similarly small rate constants were found. While the activation energy for this process is identical for both quenchers, it is much smaller than for structural fluctuations at the chromophore site as manifested by the intrinsic triplet-state lifetime. These findings are analyzed in terms of a mechanism that takes into account static quenching at large distances and does not require penetration of the quencher all the way to the chromophore.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barboy N., Feitelson J. Quenching of tryptophan phosphorescence in alcohol dehydrogenase from horse liver and its temperature dependence. Photochem Photobiol. 1985 Jan;41(1):9–13. doi: 10.1111/j.1751-1097.1985.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Bent D. V., Hayon E. Excited state chemistry of aromatic amino acids and related peptides. III. Tryptophan. J Am Chem Soc. 1975 May 14;97(10):2612–2619. doi: 10.1021/ja00843a004. [DOI] [PubMed] [Google Scholar]
- Calhoun D. B., Vanderkooi J. M., Woodrow G. V., 3rd, Englander S. W. Penetration of dioxygen into proteins studied by quenching of phosphorescence and fluorescence. Biochemistry. 1983 Mar 29;22(7):1526–1532. doi: 10.1021/bi00276a002. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. Kinetic studies of liver alcohol dehydrogenase. Biochem J. 1962 Aug;84:244–254. doi: 10.1042/bj0840244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Gratton E., Jameson D. M., Weber G., Alpert B. A model of dynamic quenching of fluorescence in globular proteins. Biophys J. 1984 Apr;45(4):789–794. doi: 10.1016/S0006-3495(84)84223-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory R. B., Lumry R. Hydrogen-exchange evidence for distinct structural classes in globular proteins. Biopolymers. 1985 Feb;24(2):301–326. doi: 10.1002/bip.360240203. [DOI] [PubMed] [Google Scholar]
- Hagaman K. A., Eftink M. R. Fluorescence quenching of Trp-314 of liver alcohol dehydrogenase by oxygen. Biophys Chem. 1984 Oct;20(3):201–207. doi: 10.1016/0301-4622(84)87024-6. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Weber G. Quenching of fluorescence by oxygen. A probe for structural fluctuations in macromolecules. Biochemistry. 1973 Oct 9;12(21):4161–4170. doi: 10.1021/bi00745a020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Weber G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry. 1973 Oct 9;12(21):4171–4179. doi: 10.1021/bi00745a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saviotti M. L., Galley W. C. Room temperature phosphorescence and the dynamic aspects of protein structure. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4154–4158. doi: 10.1073/pnas.71.10.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strambini G. B., Gabellieri E. Intrinsic phosphorescence from proteins in the solid state. Photochem Photobiol. 1984 Jun;39(6):725–729. [PubMed] [Google Scholar]
- Strambini G. B., Gonnelli M. Effects of urea and guanidine hydrochloride on the activity and dynamical structure of equine liver alcohol dehydrogenase. Biochemistry. 1986 May 6;25(9):2471–2476. doi: 10.1021/bi00357a027. [DOI] [PubMed] [Google Scholar]
- Strambini G. B. Singular oxygen effects on the room-temperature phosphorescence of alcohol dehydrogenase from horse liver. Biophys J. 1983 Jul;43(1):127–130. doi: 10.1016/S0006-3495(83)84331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subczynski W. K., Hyde J. S. Diffusion of oxygen in water and hydrocarbons using an electron spin resonance spin-label technique. Biophys J. 1984 Apr;45(4):743–748. doi: 10.1016/S0006-3495(84)84217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan W. M., Weber G. Oxygen quenching of pyrenebutyric acid fluorescence in water. A dynamic probe of the microenvironment. Biochemistry. 1970 Feb 3;9(3):464–473. doi: 10.1021/bi00805a003. [DOI] [PubMed] [Google Scholar]
- Wyckoff H. W., Handschumacher M., Murthy H. M., Sowadski J. M. The three dimensional structure of alkaline phosphatase from E. coli. Adv Enzymol Relat Areas Mol Biol. 1983;55:453–480. doi: 10.1002/9780470123010.ch6. [DOI] [PubMed] [Google Scholar]



