Abstract
We previously demonstrated that mannoprotein (MP) from Cryptococcus neoformans (CnMP) stimulates interleukin-12 production by human monocytes, thus fostering a T-helper type 1 (Th1) protective anticryptococcal response. In this paper we show that CnMP was also able to induce a Candida albicans-directed protective Th1 response. This was demonstrated for mice immunized with CnMP by induction of a delayed-type hypersensitivity (DTH) reaction to C. albicans MP (CaMP) as well as induction of gamma interferon production by CD4+ and CD8+ splenic T cells stimulated in vitro with CaMP. CnMP-immunized mice were also partially protected from lethal systemic challenge with C. albicans, as shown by prolonged median survival times and decreased fungal burden in the kidney. Much evidence supports the validity of these cross-reactive and functional Th1 responses: (i) a non-cross-reactive C. albicans antigen, such as enolase, did not produce a DTH response to CaMP; (ii) passive adoptive transfer of T cells primed with CnMP induced a DTH reaction; (iii) C. neoformans extract elicited a DTH response to CaMP; and (iv) a monoclonal antibody (7H6) directed against a major and immunodominant T-cell-stimulatory 65-kDa MP (MP65) of C. albicans also recognized discrete 100-kDa constituents of C. neoformans extracts, as well as secretory constituents of the fungus. These results suggest the presence of common Th1 antigenic determinants in the mannoproteic material of C. neoformans and C. albicans epitopes, which should be considered in devising common strategies for immunoprophylactic or immunotherapeutic control of the fungi.
Cryptococcus neoformans and Candida albicans are common causes of opportunistic fungal disease in immunocompromised or otherwise modified hosts (1). Both innate and adaptive immune responses have been shown elsewhere to contribute to host resistance to candidiasis (24), while a complex interplay between humoral and cellular immunity is critical for the control of cryptococcal infection (31).
Mannoprotein (MP) is a natural glycoconjugate usually containing between 80 and 90% mannose expressed mainly on the fungal surface and released into the external medium during growth. MPs are abundant in the cell wall of C. albicans and constitute about one-third of the dry mass (3). One of them, an immunodominant 65-kDa-MP antigen (MP65), has been extensively characterized both biochemically and immunologically (7, 8). Other MPs have been described as enzymes or adhesins involved in the pathogenicity of this fungus (10). The capsular material of C. neoformans is mainly composed of glucuronoxylomannan (galactose and xylose are minor constituents), whereas MPs are minor components of the capsule and the cell wall (6, 30).
For both organisms, MPs have long been recognized as important antigens involved in the induction of the T-cell-mediated immune response, i.e., a critical response for antifungal protection in humans. In particular, Candida and Cryptococcus MPs were shown elsewhere to induce lymphoproliferation (19, 23) and cytokine production (21, 22, 27). Previous data showed that interleukin-12 (IL-12) plays a pivotal role in induction of the T-helper type 1 (Th1) response against C. neoformans infection, this response being essential for protection (6). More recently it was demonstrated that an MP preparation of C. neoformans induces early (3 to 6 h) production of IL-12 by human monocytes in vitro, resulting in early (12 to 24 h) secretion of gamma interferon (IFN-γ) by T cells (22). Furthermore, in vivo treatment with MP induces IL-12 secretion by splenic macrophages and IL-12 p40 mRNA expression in the brain. MP-induced IL-12 and IFN-γ secretion coincides with enhanced antifungal activity, resolution of the inflammatory process, and clearance of fungal load from the brain (21).
Recent data on human monocytes stimulated in vitro by yeast or mycelial forms of C. albicans also suggest that surface-expressed MP may differentially induce IL-12, up- or down-regulating a Th1 protective cytokine pattern (5; unpublished data). When specifically examined, stimulatory properties of MPs in cell-mediated immunity (CMI) were found to be expressed by the protein rather than by the saccharide moiety of these molecules (7, 8, 18).
Because of these interesting properties, further biochemical and immunological characterization of MPs from C. albicans and C. neoformans could be extremely useful in devising immunotherapeutic or vaccination strategies against both fungi. We were particularly attracted by the possibility that protein epitopes common to C. neoformans and C. albicans MPs are involved in the induction of protective CMI. For this paper we examined this hypothesis by immunizing mice with C. neoformans MP (CnMP) and assessing C. albicans MP (CaMP)-specific CMI responses and protection from C. albicans challenge in mice.
MATERIALS AND METHODS
Mice.
Female CD1 mice purchased from Harlan Italy Laboratories (Udine, Italy) were used at 4 to 6 weeks of age.
Microorganisms.
A virulent germ-tube-forming strain of C. albicans (CA-6) isolated from a clinical specimen was used in this study. The origin, characteristics, and growth conditions of CA-6 have been described previously (2). An agerminative strain of C. albicans (PCA-2) was kindly supplied by D. Kerridge (Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom). This strain grows as a pure yeast form in vitro at 28 or at 37°C in conventional mycological media.
C. neoformans strain 6995, a thinly encapsulated strain of serotype A from the Centraalbureau voor Schimmelcultures, Delft, The Netherlands (CBS 6995 = NIH 37), was also used in this study. The cultures were maintained by serial passage on Sabouraud agar (BioMérieux, Lyon, France). In selected experiments an acapsular mutant of C. neoformans CAP67 obtained from the American Type Culture Collection (Manassas, Va.) was used. Log-phase yeasts were harvested by suspending a single colony in saline; cells were then counted on a hemocytometer and adjusted to the desired concentration. Yeasts were killed by being heated at 60°C for 30 min.
Preparation of MP extracts of C. neoformans.
An acapsular mutant of C. neoformans (NIH B-4131) was cultured in a defined medium containing 2% glucose for 5 days at 35°C as previously described (9). The culture supernatant containing MP was concentrated by ultrafiltration, and purification was performed by a combination of affinity chromatography (concanavalin A [ConA]) and anion-exchange chromatography (DEAE) (Whatman; Chemical Separation Ltd., Maidstone, England) (29). Chemically, CnMP extract used in this study consists of 13.3% protein, 72% carbohydrate, and 3.1% hexosamine (4). CnMP did not contain any contaminating ConA as analyzed by sodium dodecyl phosphate-polyacrylamide gel electrophoresis, amino acid analysis, and molecular mass determination (ConA has a molecular mass of 102 kDa). Intraperitoneal treatment with ConA (5 mg/kg of body weight, in 200 μl of saline) 6 and 24 h before C. albicans challenge did not affect survival of mice challenged with lethal doses of C. albicans or produce a delayed-type hypersensitivity (DTH) response to CaMP or CnMP challenge. This indicates that MPs had no contaminating biologically active ConA.
Preparation of MP extracts and enolase of C. albicans.
A crude MP extract was obtained from washed yeast-form C. albicans BP serotype A. MP-F2, a major MP fraction of the MP extract, was subsequently separated by ion-exchange chromatography on a DEAE Sephadex A-50 column. Only one defined batch of MP-F2 (identified as CaMP) was used for all experiments. The preparation was passed through a Sepharose-polymyxin B column (Sigma, Milan, Italy) to ensure the absence of contamination by bacterial endotoxin. A full description of the basic chemical and molecular composition of the MP-F2 fraction has been previously published (20, 26). Recombinant enolase generated as previously described (25) was used in this study as control MP-nonrelated C. albicans antigen.
Maintenance of endotoxin-free conditions.
Preparations of the various cryptococcal and candidacidal components tested negative for endotoxin contamination by a Limulus assay (Coatest endotoxin; Kabi Diagnostica, Mölndal, Sweden) with a sensitivity of 25 pg of Escherichia coli lipopolysaccharide. Nevertheless, all in vitro experiments were carried out at least once in the presence of 10 μg of polymyxin B (Sigma) per ml to neutralize any undetected contamination with bacterial lipopolysaccharide.
MAbs.
The monoclonal antibody (MAb) 7H6, which recognizes a peptide epitope of MP65, has been described previously (7, 8). It was used in immunoblots as described below. Monoclonal rat anti-mouse CD3 was obtained from BD Pharmingen (Heidelberg, Germany).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed by the Laemmli method (11) with a minigel system (mini-Protean II; Bio-Rad Laboratories, Hercules, Calif.). Samples were separated in 12% polyacrylamide gels with a 4% acrylamide stacking gel at a constant voltage of 180 V for 60 min. After electrophoresis, MPs were stained with the Bio-Rad silver staining kit or transferred electrophoretically onto polyvinylidene difluoride membranes in a Trans Blot cell (Bio-Rad). After periodate treatment, membranes were reacted with MAb 7H6. Purified MAb was used at a 1:50 dilution and detected with peroxidase-conjugated anti-mouse immunoglobulins (Boehringer Mannheim GmbH, Mannheim, Germany) at a 1:250 dilution.
Infection with C. albicans.
C. albicans yeasts from a 24-h culture on Sabouraud agar were washed with sterile endotoxin-free physiological saline (Fresenius Kabi Italia SpA, Verona, Italy); counts were determined on a hemocytometer, and the suspension was adjusted to the desired concentration for intravenous injection. The number of viable yeast cells injected was confirmed by culturing dilutions of the inoculum on Sabouraud agar. Infection was accomplished by injecting 0.5 ml of prewarmed yeast cell suspension (5 × 105 yeast cells/ml) through a 27-gauge needle into the tail vein. The outcome of infection was evaluated in terms of median survival time, number of dead animals over total number of animals, and fungal burden in the kidney expressed as CFU.
In vivo treatment.
Groups of 7 to 10 mice were injected intraperitoneally with 10 μg of CnMP in saline 24 and 6 h before infection. Mice injected with saline alone served as controls.
Clearance of Candida from kidneys.
At designated times after infection mice were sacrificed and kidneys were removed and homogenized. A serial 10-fold dilution of each sample was plated in duplicate on Sabouraud agar plates and incubated for 24 h, and CFU were counted.
Preparation and stimulation of spleen cells.
After MP treatment, spleens were aseptically removed and placed in 5 ml of RPMI 1640. Splenocytes were cultured to analyze cytokine production. A total of 20 × 106 cells/ml were cultured alone or in the presence of CnMP or CaMP (2.5 μg/ml), heat-inactivated C. neoformans (20 × 106 cells/ml), or heat-inactivated C. albicans (20 × 106 cells/ml) in RPMI-10% fetal calf serum. As a positive control, cells were stimulated with 50 ng of phorbol 12-myristate 13-acetate (PMA; Sigma)/ml and 2 mM ionomycin (Sigma). After 18 h of incubation, intracellular IFN-γ was measured. In parallel experiments, after 7 days the supernatants were assayed for IFN-γ content.
Determination of IFN-γ production.
Cytokine levels in culture supernatant fluids were measured with an enzyme-linked immunosorbent assay kit for mouse IFN-γ (Endogen Inc., Woburn, Mass.).
Intracellular IFN-γ assay.
The intracellular IFN-γ in CD4 or CD8 T cells was determined with an intracellular staining kit (IC Screen) provided by Biosource International (Camarillo, Calif.). Stimulations were carried out in the presence of the transport inhibitor monensin, which prevents the release of IFN-γ into the extracellular milieu. The stimulated cells were stained with fluorescein isothiocyanate-tagged anti-CD4 or anti-CD8 MAb and fixed with paraformaldehyde. Cells were stained with an R-phycoerythrin-labeled anti-IFN-γ antibody in the presence of a permeabilizing agent. The stained cells were then analyzed by flow cytometry for the coexistence of two different fluorescent tags in the same cell.
Induction and elicitation of the DTH response.
Mice were intraperitoneally injected twice in 24 h with 200 μl of saline or 10 μg of CnMP in saline. After 8 days the left footpads were injected with 30 μl (10 μg) of CnMP, CaMP, or extracts from the acapsular strain (7698) and the right footpad was injected with 30 μl of saline. The DTH reaction was recorded 24 h later by weighing the footpads as a measure of swelling, and the results were expressed as the increase in weight of the left hind footpad over that of the saline-injected right hind counterpart. Data were expressed as means ± standard errors of the means (SEM) of five mice per group per experiment.
Adoptive transfer of T cells.
Mice were injected with 10 μg of CnMP twice in 24 h. T cells were purified from spleens as described previously (15) 7 days after treatment and restimulated in vitro with CnMP for 24 h. These cells (more than 95% CD3 positive as revealed by flow cytometric analysis) were injected at the dose of 106 into naïve mice that had been challenged in the footpad with CaMP or saline. The DTH reaction was recorded 24 h later.
Statistical analysis.
Statistical significance between groups was analyzed by the Mann-Whitney U test. Survival of the different groups of mice in the protection experiments was compared by the log-rank survival test. For in vitro experiments statistical analysis was performed by the analysis of variance test. A P value of <0.05 was considered significant.
RESULTS
In consideration of the previously published evidence that both CnMP and CaMP could induce and detect specific CMI responses (16, 17), we explored the possibility of a cross-reactive antigenic response to the two fungi by immunizing mice with CnMP and then assessing DTH reaction and IFN-γ production by splenic lymphocytes stimulated in vitro with CnMP or CaMP.
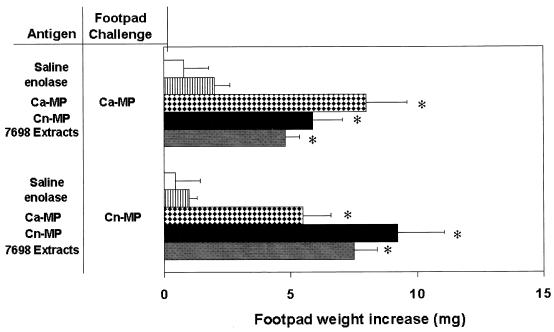
As expected, mice immunized with CnMP generated a strong DTH reaction when the footpad was challenged 7 days posttreatment with the corresponding antigen, compared to that of untreated control mice. Interestingly, a marked footpad swelling was also detected in mice injected with material from C. neoformans, such as 7698 extracts and CnMP and DTH tested with CaMP (Fig. 1). The difference between the two reactions (against CnMP and CaMP) was not statistically significant. The reaction specificity was confirmed by the fact that a non-cross-reactive antigen, enolase, did not induce a DTH response when used instead of CaMP.
FIG. 1.
DTH responses elicited by CnMP or CaMP in mice treated 8 days earlier with CnMP, CaMP, or 7698 extracts. Data are representative of three experiments with five mice per group per experiment. Data are means ± standard deviations. ∗, P < 0.05 (CnMP-treated versus saline-treated group).
The cross-reactive immune response between CaMP and CnMP was also demonstrated by the fact that reverse treatment with CaMP induced a DTH response against CnMP similar to that obtained with the corresponding antigen (CnMP) (Fig. 1).
To verify whether T cells primed with CnMP were indeed responsible for DTH induction, splenic T cells (106) from naïve or CnMP-treated mice (6 days posttreatment) were adoptively transferred intravenously into naïve mice 1 day before CaMP challenge. The results showed that T cells from CnMP-treated mice produced a significant increase of 5.6 ± 0.7 mg in footpad weight (P < 0.05, CnMP treated versus saline treated). Conversely, a nonsignificant difference was observed in mice receiving T cells from naïve mice (increase of footpad weight was 1.9 ± 0.5 mg).
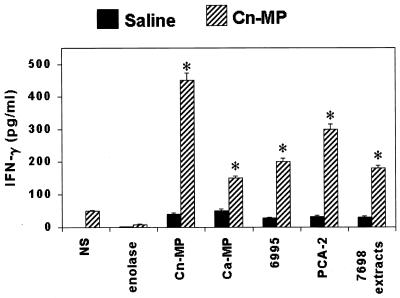
Since IFN-γ is critically involved in the generation and expression of the DTH reaction, we examined whether, and to what extent, this cytokine was produced in the supernatants of splenocyte cultures from mice immunized with CnMP and stimulated in vitro with CnMP or CaMP or other control stimuli. Figure 2 shows that, as early as 1 day after in vitro stimulation, splenocytes from CnMP-immunized animals, but not from nonimmune mice, produced elevated quantities of the cytokine (from about 150 to more than 450 pg/ml) in response to in vitro treatments not only with the specific immunogens but also with CaMP and whole inactivated C. albicans cells (PCA-2). Interestingly, in vitro stimulation with whole cells of the fungus was the same, if not superior, in terms of cytokine production as stimulation of whole C. neoformans (6995) (Fig. 2). In addition, appreciable levels of IFN-γ were observed upon stimulation with extracts from C. neoformans cells (7698). Conversely, a non-cross-reactive unrelated antigen, enolase, did not induce IFN-γ (Fig. 2). A substantial drop in IFN-γ production (no more than 25 pg/ml) was observed when the cytokine was sought in splenocyte supernatant cultures from mice treated 7 days before with CnMP. In this case, no significant difference was found between the low, spontaneous cytokine production of unstimulated cells and that of the cells stimulated by C. neoformans or C. albicans materials (data not shown)
FIG. 2.
Effect of CnMP on IFN-γ production by total splenocytes. Mice were treated twice with saline or 10 μg of CnMP and sacrificed 1 day later. Splenocytes (20 × 106) were recovered and cultured alone or in the presence of CnMP (2.5 μg/ml), CaMP (2.5 μg/ml), heat-inactivated C. neoformans (40 × 106 cells/ml), heat-inactivated C. albicans (40 × 106 cells/ml), 7698 extracts (5 μg/ml), or enolase (5 μg/ml) in RPMI-10% fetal calf serum for 1 day. IFN-γ levels in culture supernatants were evaluated by enzyme-linked immunosorbent assay. The results are means ± SEM of three separate experiments. NS, not stimulated. ∗, P < 0.05 (CnMP-treated versus saline-treated mice).
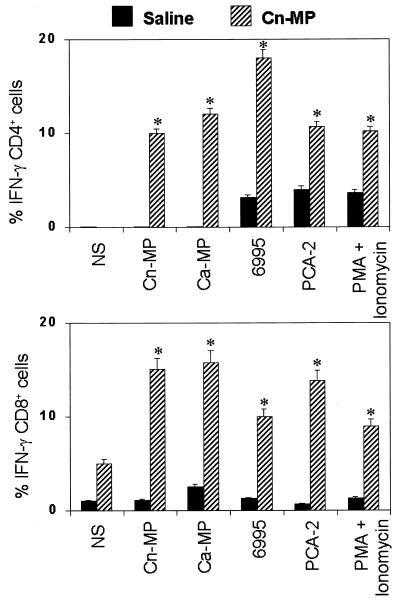
Knowing that IFN-γ may be secreted by CD4 or CD8 T cells and that CD8 could also be involved in antifungal protection (1), we determined the percentage of IFN-γ-producing CD4 and CD8 cells over the total number of splenic lymphocytes. As shown in Fig. 3, a substantial proportion (approximately 15 to 20%) of both CD4 and CD8 splenic lymphocytes of mice immunized with CnMP produced IFN-γ in the presence of both cryptococcal and candidal materials. In particular, this production was not inferior to that of the positive control with PMA plus ionomycin-treated cells. On average, about 5% of the CD8 T cells from immune animals were IFN-γ positive and the percentage increased about threefold upon in vitro stimulation (Fig. 3). No statistically significant difference was found in the percentage of CD4 or CD8 cells positive for intracellular IFN-γ among the various cultures stimulated with cryptococcal or candidal materials. Even though the percentages of cytokine-producing CD4 and CD8 cells were similar, it is likely that the major contribution to IFN-γ production may be ascribed to CD4+ cells because they represent more than 65% of total T cells among splenocytes of CnMP-treated mice.
FIG. 3.
Effect of CnMP on IFN-γ synthesis from splenic T cells. Mice were treated twice with saline or CnMP (10 μg) and sacrificed 24 h later. Splenocytes (20 × 106) were recovered and cultured alone or in the presence of CnMP (2.5 μg/ml), CaMP (2.5 μg/ml), heat-inactivated C. neoformans (6995, 40 × 106 cells/ml), or heat-inactivated C. albicans (PCA-2, 40 × 106 cells/ml) for 18 h. As a positive control, cells were stimulated with 50 ng of PMA/ml and 2 mM ionomycin. Stimulations were carried out in the presence of monensin. The stimulated cells were stained with fluorescein isothiocyanate-labeled anti-CD4 or anti-CD8 MAb and fixed with paraformaldehyde. Cells were then stained with an R-phycoerythrin-labeled anti-IFN-γ antibody in the presence of a permeabilizing agent. The stained cells were analyzed by flow cytometry for the coexistence of two different fluorescent tags in the same cell. Data are expressed as the percentages of CD4 IFN-γ (upper panel)- or CD8 IFN-γ (lower panel)-positive cells. Results represent means ± SEM of three separate experiments.∗, P < 0.05 (CnMP-treated versus saline-treated mice).
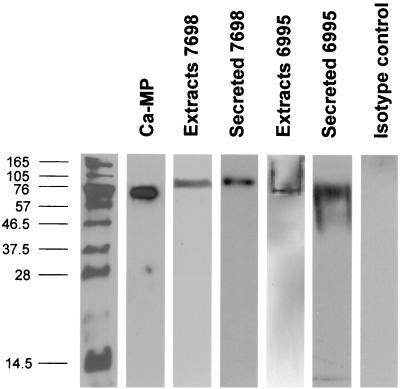
Given that CnMP is able to induce a specific T-cell response to CaMP, and the major antigenic component of CaMP capable of inducing a T-cell response is a 65-kDa MP (MP65) (7), we hypothesized that CnMP could share common epitopes with MP65. Thus, we verified the ability of a MAb specific for a protein epitope of the N-terminal moiety of MP65 (MAb 7H6) (A. Cassone, unpublished observations) to recognize CnMP and/or other cryptococcal components present in cellular extracts or secreted by the fungus. Experiments with CnMP showed no reaction with MAb 7H6. However, other extracts of C. neoformans and CaMP were indeed recognized by the antibody (Fig. 4). C. neoformans extract demonstrated a well-defined, discrete reactivity with a molecular constituent of about 100 kDa, both for the acapsular and for the encapsulated fungus. Reactivity with the secretory materials of the encapsulated strain (6995) was less defined and more polydisperse than in the extract (Fig. 4).
FIG. 4.
Western blotting with MAb 7H6 of CaMP or total cell extracts and secreted material of acapsular (7698) and encapsulated (6995) C. neoformans. Western blot analysis with MAb 7H6 was performed on total cell extracts and secreted materials as described in Materials and Methods. Yeasts were cultured in RPMI at 30°C for 72 h. Total protein loaded amounts were ∼10 μg/lane for all samples. The molecular mass markers in kilodaltons are shown at the left.
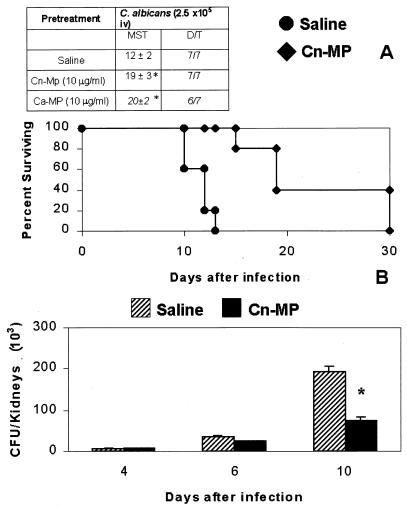
Finally, in view of the report that treatment with selected MP fractions of C. albicans induced partial protection against systemic infection by this fungus (owing to the activation of a Th1-type response) (16), we examined mice immunized with CnMP to determine if they were also able to resist systemic challenge by C. albicans. To this end, the animals were injected intraperitoneally with CnMP (10 μg/200 μl) 24 and 6 h before intravenous challenge with C. albicans (2.5 × 105 cells/0.5 ml), and animal survival and fungal CFU were determined. As shown in Fig. 5A, survival of mice immunized with CnMP was significantly higher than that of nonimmune animals, corresponding to the statistically significant reduction of the Candida burden in the kidney (Fig. 5B). Similar results in terms of survival were obtained in our experimental system by treatment with the corresponding antigen CaMP (Fig. 5A).
FIG. 5.
(A) Survival of mice after intravenous (iv) challenge with a lethal dose of viable C. albicans. Mice were treated with saline or 10 μg of CnMP in saline 24 and 6 h before challenge. (Inset) Survival of mice immunized with C. albicans materials and challenged with 2 × 105 C. albicans cells. The results are reported as median survival time (MST) and as number of dead animals over total number of animals tested (D/T). ∗, P < 0.05 (CnMP- or CaMP-treated versus saline-treated mice). (B) Effect of CnMP on viable C. albicans cells in the kidneys. Mice were treated with saline or 10 μg of CnMP 24 and 6 h before challenge with C. albicans (2.5 × 105 cells/mouse). At different times, mice were killed and viable yeast cells in the kidneys (CFU) were counted by plating samples of homogenized tissue on Sabouraud agar. Data are means ± standard deviations for four or five mice. ∗, P < 0.05 (CnMP-treated versus saline-treated mice).
The protective effect of CnMP was also evaluated against the homologous strain of C. neoformans (CAP67). Given that acapsular C. neoformans CAP67 is unable to produce lethal infection in mice, we evaluated CFU from brains 7 days after CAP67 (5 × 106) intravenous challenge. The results showed that CnMP treatment produced a reduction of C. neoformans load in the brain as evaluated by CFU recovery (data not shown).
DISCUSSION
The results reported here demonstrate that MPs from C. neoformans and C. albicans share common antigenic determinants capable of eliciting potentially protective Th1-type responses in immunized mice. This assertion derives from three lines of evidence. First, animals primed with CnMP generated a DTH response against CaMP to a degree that did not differ substantially from the response to the priming antigen CnMP. This response was immune mediated as shown by T-cell adoptive transfer. Second, splenocytes of mice immunized with CnMP produced IFN-γ when cultured in vitro with CaMP. Third, IFN-γ-producing CD4 and CD8 T cells were markedly increased in animals immunized with CnMP. CD4 and CD8 cell expansion upon in vitro stimulation with CaMP was comparable to that observed with the priming antigen and also similar to that detected with whole C. albicans and C. neoformans as stimulants. Finally, the cross-reactive T-cell response was functional in terms of protection against C. albicans and C. neoformans.
As a separate yet connected piece of evidence for the sharing of antigenic MP determinants between the two fungi, we show here that MAb 7H6 recognizes a peptide epitope close to the N-terminal moiety of a major immunodominant T-cell immunogen of C. albicans MP65 (7; A. Cassone, unpublished data) and reacts with defined molecular constituents of C. neoformans extracts, suggesting that a homologous molecular constituent may be present in CnMPs. This is consistent with the ability of C. neoformans extracts to elicit a specific DTH response against C. albicans. The lack of reaction of MAb 7H6 with CnMP may not necessarily predict the absence of a MAb-reactive epitope also in CnMP, as it could have become unreactive during the process of CnMP preparation, which includes heating at 100°C for 5 min. If so, this epitope may be present in a molecule that is smaller than C. albicans MP65, since the calculated molecular mass of CnMP is about 8.2 kDa (4). Our data, in particular the strong reaction of MAb 7H6 with a 75-kDa constituent of C. neoformans extract, may also imply that CnMP is a degradation product of this or another polydisperse MAb-reactive extract or secretory material of C. neoformans. Studies of the molecular cloning of the MP65-encoding gene of C. albicans are in progress (12).
MP molecules have long been argued to be important constituents for the pathogen-host relationship in both C. neoformans and C. albicans. MP65 of C. albicans has been shown elsewhere to possess strong immunogenic properties, including DTH elicitation (16) and induction of T-cell responses (23). It has been recognized to contain dominant T-cell epitopes in humans (12) and is used to expand specific T cells, in particular CD4+ T-cell clones producing IFN-γ (18). Partial protection against systemic challenge by C. albicans in mice has also been attributed to an anti-MP65 response (16).
MPs of C. neoformans have been identified elsewhere as the major antigens inducing CMI responses to C. neoformans (17), including DTH elicitation, T-cell activation, and cytokine production by peripheral blood mononuclear cells (4, 19, 22). More recently the molecular characterization of a C. neoformans MP that stimulates a T-cell response has been provided (13, 14). We have shown that our MP preparation from C. neoformans is capable of inducing IL-12 production from human monocytes and protection against a lethal fungus challenge (21, 22).
Thus, MPs from C. albicans and C. neoformans appear to play an important role in the elicitation of protective antifungal responses. The data provided in this paper not only strengthen this concept but also suggest the involvement of a common determinant in these molecules. The specificity of the T-cell response has been underlined by the inability of MP to affect the blastogenic response to mitogens, such as ConA. More evidence on the specific immune response elicited by MP from C. neoformans has been provided by the induction of IFN-γ secretion from CD4 and CD8 T cells. It is noteworthy that there is a profound difference between IFN-γ recovered after 1 day and that recovered after 7 days. Peak IFN-γ production occurring as early as 1 day after stimulation is consistent with the fact that MP induces an early and massive production of IL-12 after 6 h (22). Hence, the abundant production after 1 day of culture likely reflects this event, since IL-12 and IFN-γ production are closely related (28). This is consistent with the fact that MP from C. albicans stimulates CD4 T cells (18) and that CD4 and CD8 lymphocytes are important in controlling C. albicans infection (1). The strategy of the immune system in mounting a specific response against C. albicans indicates that MP from C. neoformans contains common epitopes potentially acting as stimulators of a protective response to the fungi. This notion is supported by immunoblotting reactions with a MAb to C. albicans MP65: reactive bands were observed when extracts from C. neoformans acapsular strains were used.
However, we should consider that the observed protection might be due to multiple effects including the stimulation of the innate immune system that could occur through mannose or T-cell-like receptors, at least in the early phase of the immune response. Nevertheless, we can attribute a role to the specific immune response because (i) the non-cross-reactive antigen enolase did not produce a DTH response or affect survival and (ii) adoptive transfer of T cells from CnMP-treated mice induced a DTH response. Thus, MP2 protection may provide early stimulation of a nonspecific immune response with consequent stimulation of specific immunity.
Overall, the evidence for common MP epitopes in C. neoformans and C. albicans suggests at least two considerations: first, that the two fungi synthesize and secrete MP with a potentially similar positive impact on the immune system, and second, that the specific T-cell response could be stimulated, at least in part, by different MPs sharing common epitopes Therefore, identification of the epitopes involved in fungal CMI induction strongly suggests the possibility of using a common protein antigen to elicit a potentially protective adaptive immune response against the fungi.
Acknowledgments
We thank Jo-Anne Rowe for excellent secretarial and editorial support.
This study was supported by a grant from the National Research Program on AIDS, “Opportunistic Infections and Tuberculosis,” contract no. 50D.31, Italy.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Ashman, R. B. 1998. Candida albicans: pathogenesis, immunity and host defence. Res. Immunol. 149:281-288. [DOI] [PubMed] [Google Scholar]
- 2.Baccarini, M., A. Vecchiarelli, A. Cassone, and F. Bistoni. 1985. Killing of yeast, germ-tube and mycelial forms of Candida albicans by murine effectors as measured by a radiolabel release microassay. J. Gen. Microbiol. 131:505-513. [DOI] [PubMed] [Google Scholar]
- 3.Cassone, A., and A. Torosantucci. 1991. Candida albicans. Springer-Verlag, Berlin, Germany.
- 4.Chaka, W., A. F. Verheul, V. V. Vaishnav, R. Cherniak, J. Scharringa, J. Verhoef, H. Snippe, and I. M. Hoepelman. 1997. Cryptococcus neoformans and cryptococcal glucuronoxylomannan, galactoxylomannan, and mannoprotein induce different levels of tumor necrosis factor alpha in human peripheral blood mononuclear cells. Infect. Immun. 65:272-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiani, P., C. Bromuro, and A. Torosantucci. 2000. Defective induction of interleukin-12 in human monocytes by germ-tube forms of Candida albicans. Infect. Immun. 68:5628-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decken, K., G. Kohler, K. Palmer-Lehmann, A. Wunderlin, F. Mattner, J. Magram, M. K. Gately, and G. Alber. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 66:4994-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez, M. J., B. Maras, A. Barca, R. La Valle, D. Barra, and A. Cassone. 2000. Biochemical and immunological characterization of MP65, a major mannoprotein antigen of the opportunistic human pathogen Candida albicans. Infect. Immun. 68:694-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez, M. J., A. Torosantucci, S. Arancia, B. Maras, L. Parisi, and A. Cassone. 1996. Purification and biochemical characterization of a 65-kilodalton mannoprotein (MP65), a main target of anti-Candida cell-mediated immune responses in humans. Infect. Immun. 64:2577-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James, P. G., and R. Cherniak. 1992. Galactoxylomannans of Cryptococcus neoformans. Infect. Immun. 60:1084-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapteyn, J. C., R. C. Montijn, G. J. Dijkgraaf, H. Van den Ende, and F. M. Klis. 1995. Covalent association of β-1,3-glucan with β-1,6-glucosylated mannoproteins in cell walls of Candida albicans. J. Bacteriol. 177:3788-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 12.La Valle, R., S. Sandini, M. J. Gomez, F. Mondello, G. Romagnoli, R. Nisini, and A. Cassone. 2000. Generation of a recombinant 65-kilodalton mannoprotein, a major antigen target of cell-mediated immune response to Candida albicans. Infect. Immun. 68:6777-6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitz, S. M., S. Nong, M. K. Mansour, C. Huang, and C. A. Specht. 2001. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. Proc. Natl. Acad. Sci. USA 98:10422-10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansour, M. K., L. S. Schlesinger, and S. M. Levitz. 2002. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J. Immunol. 168:2872-2879. [DOI] [PubMed] [Google Scholar]
- 15.Mencacci, A., R. Spaccapelo, G. Del Sero, K. H. Enssle, A. Cassone, F. Bistoni, and L. Romani. 1996. CD4+ T-helper-cell responses in mice with low-level Candida albicans infection. Infect. Immun. 64:4907-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mencacci, A., A. Torosantucci, R. Spaccapelo, L. Romani, F. Bistoni, and A. Cassone. 1994. A mannoprotein constituent of Candida albicans that elicits different levels of delayed-type hypersensitivity, cytokine production, and anticandidal protection in mice. Infect. Immun. 62:5353-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy, J. W. 1988. Influence of cryptococcal antigens on cell-mediated immunity. Rev. Infect. Dis. 10(Suppl. 2):S432-S435. [DOI] [PubMed] [Google Scholar]
- 18.Nisini, R., G. Romagnoli, M. J. Gomez, R. La Valle, A. Torosantucci, S. Mariotti, R. Teloni, and A. Cassone. 2001. Antigenic properties and processing requirements of 65-kilodalton mannoprotein, a major antigen target of anti-Candida human T-cell response, as disclosed by specific human T-cell clones. Infect. Immun. 69:3728-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orendi, J. M., A. F. Verheul, N. M. De Vos, M. R. Visser, H. Snippe, R. Cherniak, V. V. Vaishnav, G. T. Rijkers, and J. Verhoef. 1997. Mannoproteins of Cryptococcus neoformans induce proliferative response in human peripheral blood mononuclear cells (PBMC) and enhance HIV-1 replication. Clin. Exp. Immunol. 107:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palma, C., D. Serbousek, A. Torosantucci, A. Cassone, and J. Y. Djeu. 1992. Identification of a mannoprotein fraction from Candida albicans that enhances human polymorphonuclear leukocyte (PMNL) functions and stimulates lactoferrin in PMNL inhibition of candidal growth. J. Infect. Dis. 166:1103-1112. [DOI] [PubMed] [Google Scholar]
- 21.Pietrella, D., R. Cherniak, C. Strappini, S. Perito, P. Mosci, F. Bistoni, and A. Vecchiarelli. 2001. Role of mannoprotein in induction and regulation of immunity to Cryptococcus neoformans. Infect. Immun. 69:2808-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitzurra, L., R. Cherniak, M. Giammarioli, S. Perito, F. Bistoni, and A. Vecchiarelli. 2000. Early induction of interleukin-12 by human monocytes exposed to Cryptococcus neoformans mannoproteins. Infect. Immun. 68:558-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinti, I., C. Palma, E. C. Guerra, M. J. Gomez, I. Mezzaroma, F. Aiuti, and A. Cassone. 1991. Proliferative and cytotoxic responses to mannoproteins of Candida albicans by peripheral blood lymphocytes of HIV-infected subjects. Clin. Exp. Immunol. 85:485-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romani, L. 2000. Innate and adaptive immunity in Candida albicans infections and saprophytism. J. Leukoc. Biol. 68:175-179. [PubMed] [Google Scholar]
- 25.Sandini, S. M. R., S. Arancia, M. J. Gomez, and R. La Valle. 1999. Generation of a highly immunogenic recombinant enolase of the human opportunistic pathogen Candida albicans. Biotechnol. Appl. Biochem. 29:223-227. [PubMed] [Google Scholar]
- 26.Torosantucci, A., C. Bromuro, M. J. Gomez, C. M. Ausiello, F. Urbani, and A. Cassone. 1993. Identification of a 65-kDa mannoprotein as a main target of human cell-mediated immune response to Candida albicans. J. Infect. Dis. 168:427-435. [DOI] [PubMed] [Google Scholar]
- 27.Torosantucci, A., C. Palma, M. Boccanera, C. M. Ausiello, G. C. Spagnoli, and A. Cassone. 1990. Lymphoproliferative and cytotoxic responses of human peripheral blood mononuclear cells to mannoprotein constituents of Candida albicans. J. Gen. Microbiol. 136:2155-2163. [DOI] [PubMed] [Google Scholar]
- 28.Trinchieri, G. 1998. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv. Immunol. 70:83-243. [DOI] [PubMed] [Google Scholar]
- 29.Turner, S. H., R. Cherniak, and E. Reiss. 1984. Fractionation and characterization of galactoxylomannan from Cryptococcus neoformans. Carbohydr. Res. 125:343-349. [DOI] [PubMed] [Google Scholar]
- 30.Vecchiarelli, A. 2000. Immunoregulation by capsular components of Cryptococcus neoformans. Med. Mycol. 38:407-417. [DOI] [PubMed] [Google Scholar]
- 31.Vecchiarelli, A., and A. Casadevall. 1998. Antibody-mediated effects against Cryptococcus neoformans: evidence for interdependency and collaboration between humoral and cellular immunity. Res. Immunol. 149:321-333. [DOI] [PubMed] [Google Scholar]