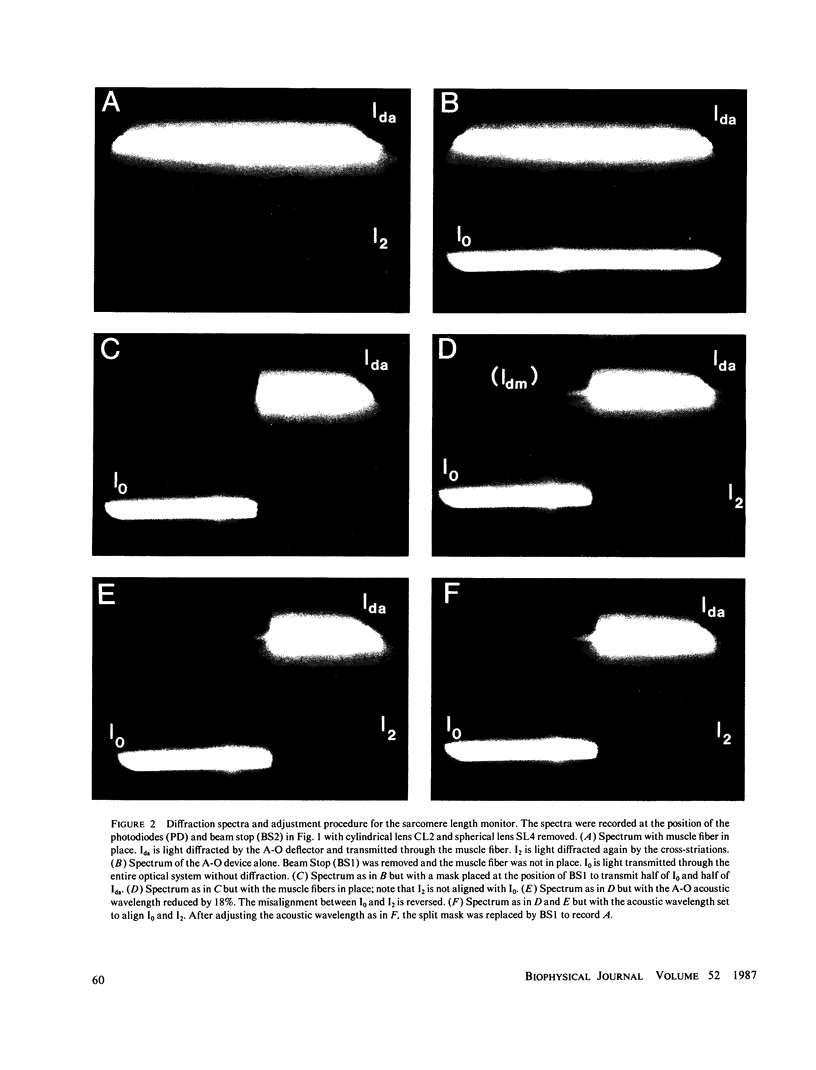
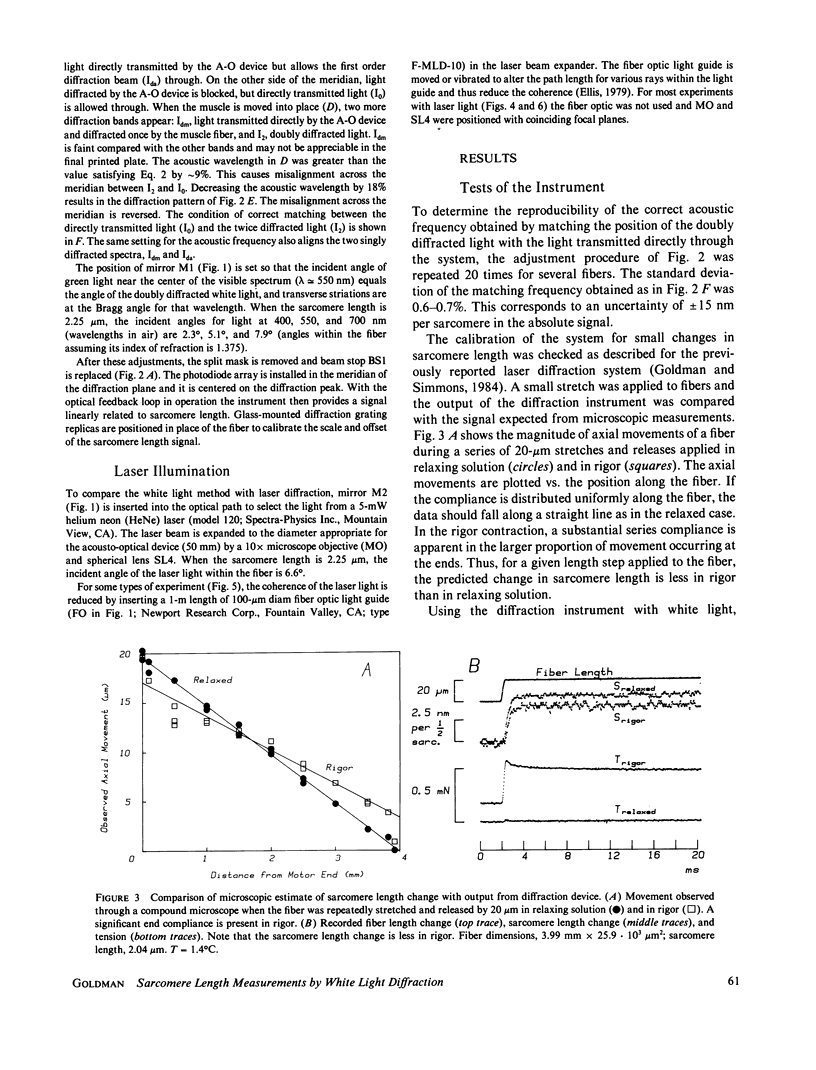
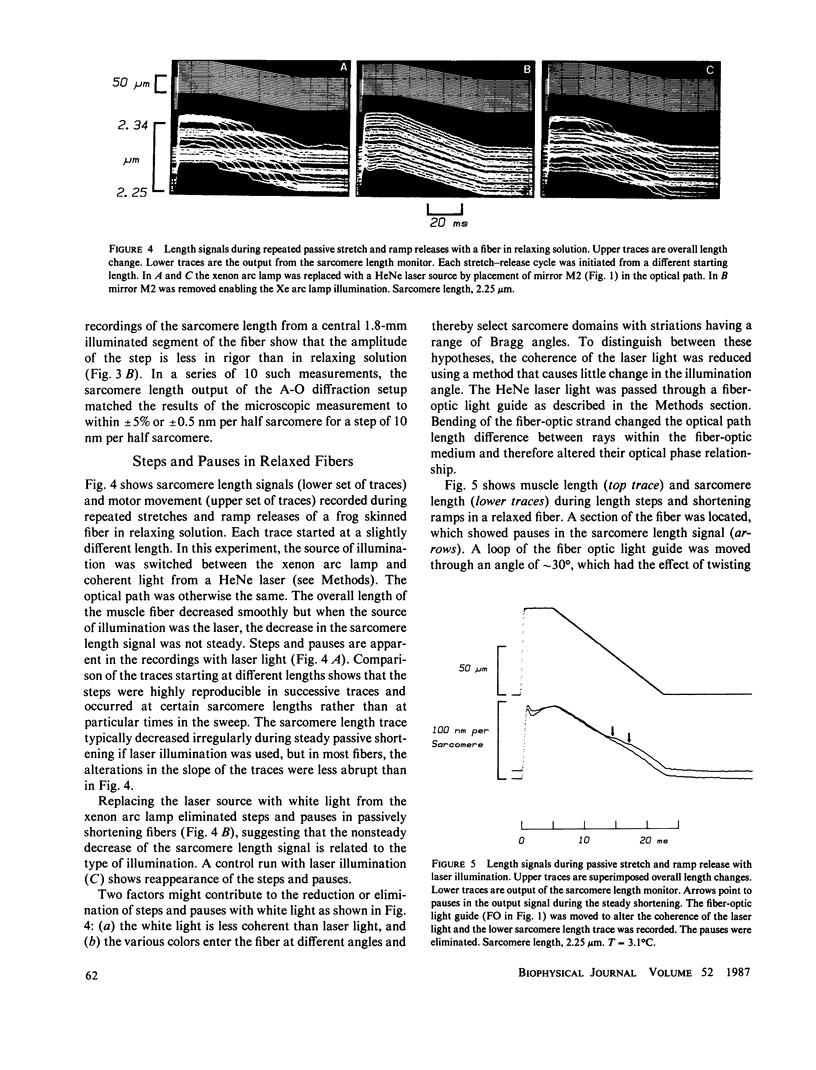
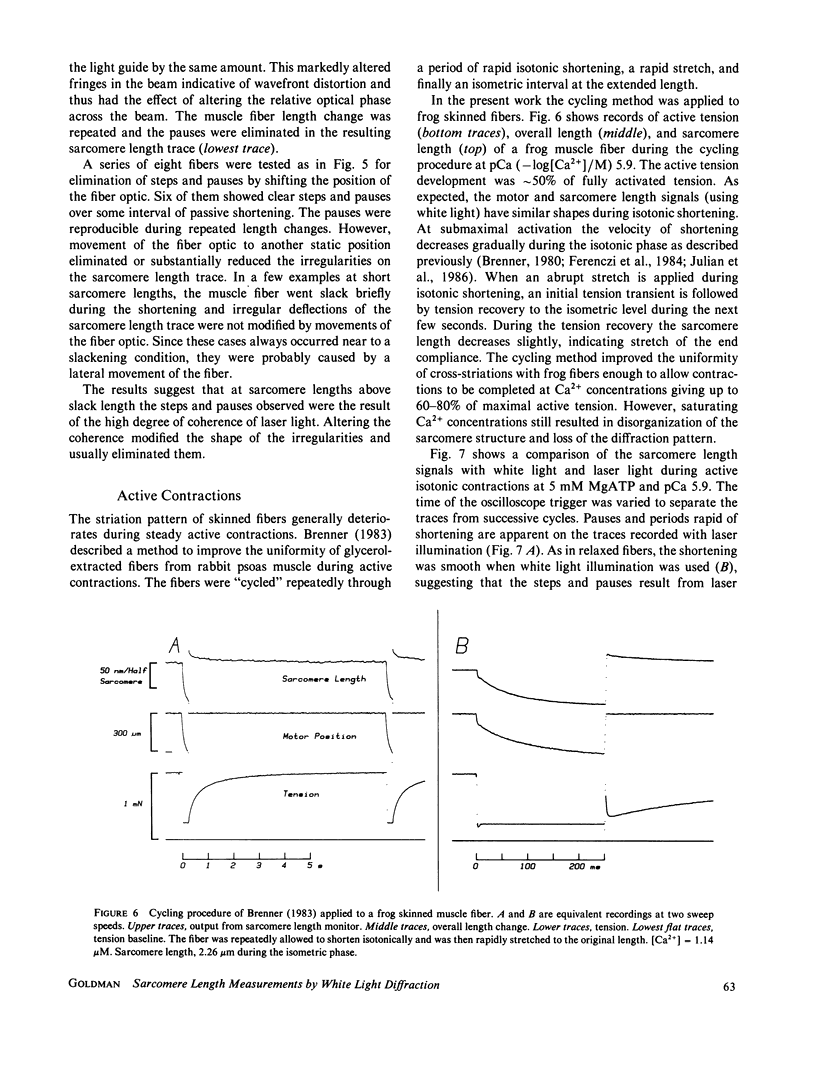
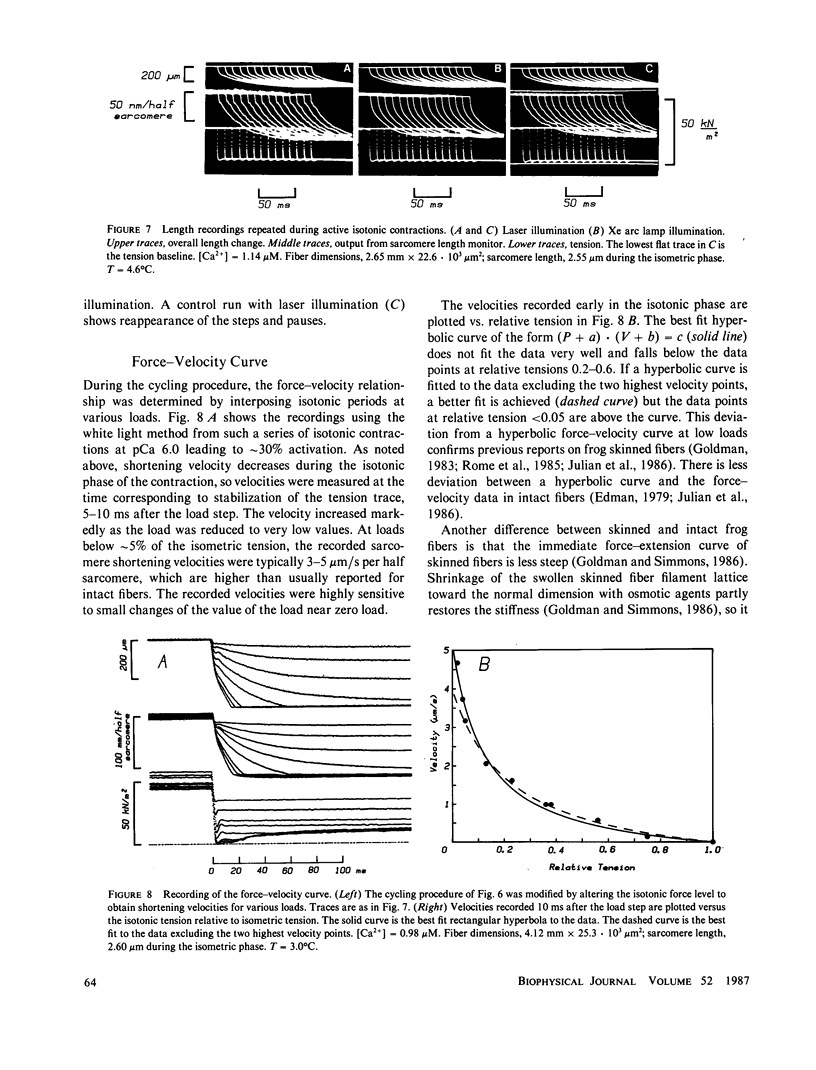
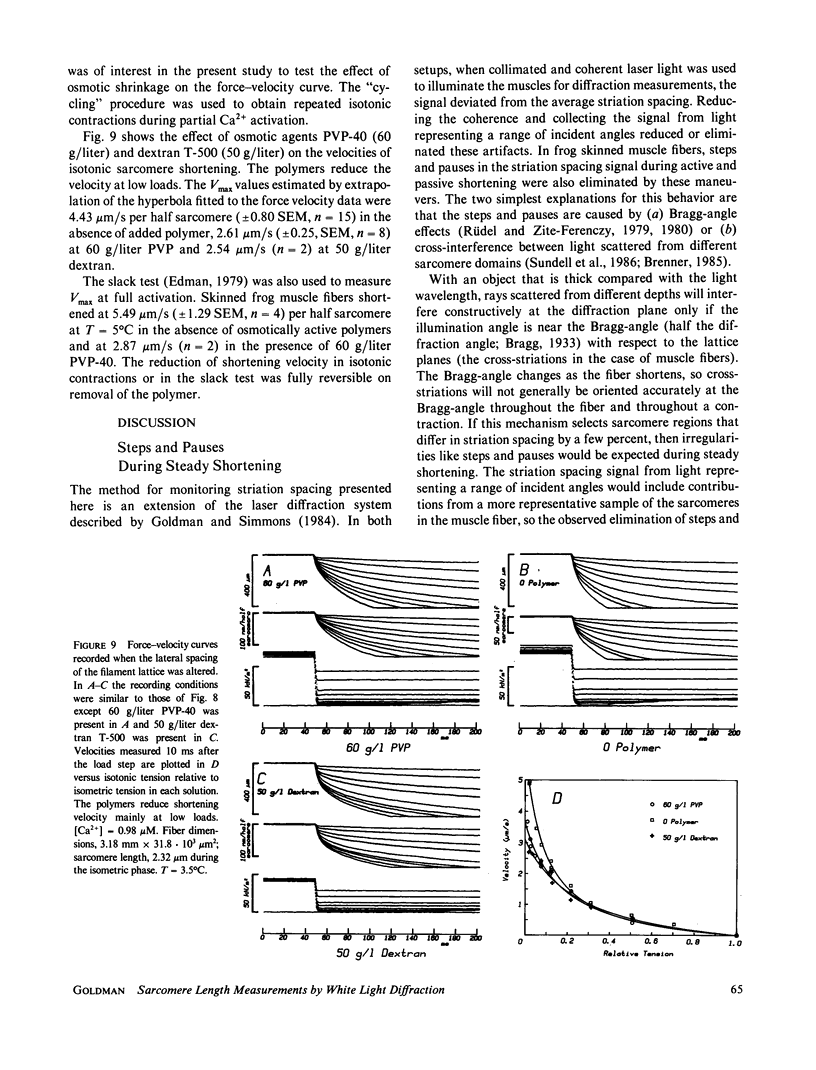
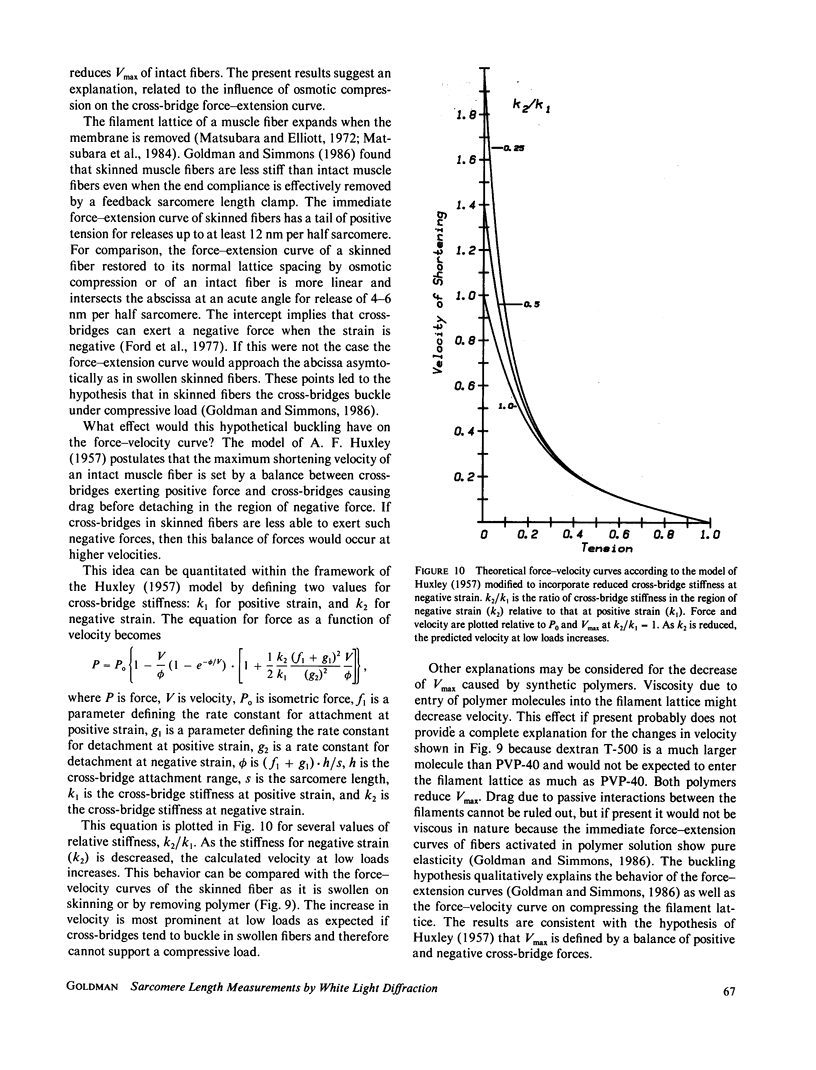
Abstract
A new optical-electronic method has been developed to detect striation spacing of single muscle fibers. The technique avoids Bragg-angle and interference-fringe effects associated with laser light diffraction by using polychromatic (white) light. The light is diffracted once by an acousto-optical device and then diffracted again by the muscle fiber. The double diffraction reverses the chromatic dispersion normally obtained with polychromatic light. In frog skinned muscle fibers, active and passive sarcomere shortening were smooth when observed by white light diffraction, whereas steps and pauses occurred in the striation spacing signals obtained with laser illumination. During active contractions skinned fibers shortened at high rates (3-5 microns/s per half sarcomere, 0-5 degrees C) at loads below 5% of isometric tension. Compression of the myofibrillar lateral filament spacing using osmotic agents reduced the shortening velocity at low loads. A hypothesis is presented that high shortening velocities are observed with skinned muscle fibers because the cross-bridges cannot support compressive loads when the filament lattice is swollen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altringham J. D., Bottinelli R., Lacktis J. W. Is stepwise sarcomere shortening an artefact? Nature. 1984 Feb 16;307(5952):653–655. doi: 10.1038/307653a0. [DOI] [PubMed] [Google Scholar]
- Brenner B. Sarcomeric domain organization within single skinned rabbit psoas fibers and its effects on laser light diffraction patterns. Biophys J. 1985 Dec;48(6):967–982. doi: 10.1016/S0006-3495(85)83860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys J. 1983 Jan;41(1):99–102. doi: 10.1016/S0006-3495(83)84411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozovich F. V., Pollack G. H. Muscle contraction generates discrete sound bursts. Biophys J. 1983 Jan;41(1):35–40. doi: 10.1016/S0006-3495(83)84403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzig J. A., Goldman Y. E. Suppression of muscle contraction by vanadate. Mechanical and ligand binding studies on glycerol-extracted rabbit fibers. J Gen Physiol. 1985 Sep;86(3):305–327. doi: 10.1085/jgp.86.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay M. J., Ishide N., Jacobson R. C., Pollack G. H., Tirosh R. Stepwise sarcomere shortening: analysis by high-speed cinemicrography. Science. 1981 Sep 25;213(4515):1523–1525. doi: 10.1126/science.7280674. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Goldman Y. E., Simmons R. M. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J Physiol. 1984 May;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5'-triphosphate. J Physiol. 1984 Sep;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Control of sarcomere length in skinned muscle fibres of Rana temporaria during mechanical transients. J Physiol. 1984 May;350:497–518. doi: 10.1113/jphysiol.1984.sp015215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. The stiffness of frog skinned muscle fibres at altered lateral filament spacing. J Physiol. 1986 Sep;378:175–194. doi: 10.1113/jphysiol.1986.sp016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H. L., Pollack G. H. Stepwise shortening in unstimulated frog skeletal muscle fibres. J Physiol. 1985 May;362:173–188. doi: 10.1113/jphysiol.1985.sp015669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Babu A. Intrinsic shortening speed of temperature-jump-activated intact muscle fibers. Effects of varying osmotic pressure with sucrose and KCl. Biophys J. 1984 Feb;45(2):431–445. doi: 10.1016/S0006-3495(84)84166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres at lengths above the optimum. J Physiol. 1980 Jul;304:529–539. doi: 10.1113/jphysiol.1980.sp013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol. 1986 Jan;370:181–199. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Elliott G. F. X-ray diffraction studies on skinned single fibres of frog skeletal muscle. J Mol Biol. 1972 Dec 30;72(3):657–669. doi: 10.1016/0022-2836(72)90183-0. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Goldman Y. E., Simmons R. M. Changes in the lateral filament spacing of skinned muscle fibres when cross-bridges attach. J Mol Biol. 1984 Feb 15;173(1):15–33. doi: 10.1016/0022-2836(84)90401-7. [DOI] [PubMed] [Google Scholar]
- Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979 Jul;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack G. H., Iwazumi T., ter Keurs H. E., Shibata E. F. Sarcomere shortening in striated muscle occurs in stepwise fashion. Nature. 1977 Aug 25;268(5622):757–759. doi: 10.1038/268757a0. [DOI] [PubMed] [Google Scholar]
- Pollack G. H. Quantal mechanisms in cardiac contraction. Circ Res. 1986 Jul;59(1):1–8. doi: 10.1161/01.res.59.1.1. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Zite-Ferenczy F. Do laser diffraction studies on striated muscle indicate stepwise sarcomere shortening? Nature. 1979 Apr 5;278(5704):573–575. doi: 10.1038/278573a0. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Zite-Ferenczy F. Efficiency of light diffraction by cross-striated muscle fibers under stretch and during isometric contraction. Biophys J. 1980 Jun;30(3):507–516. doi: 10.1016/S0006-3495(80)85110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundell C. L., Goldman Y. E., Peachey L. D. Fine structure in near-field and far-field laser diffraction patterns from skeletal muscle fibers. Biophys J. 1986 Feb;49(2):521–530. doi: 10.1016/S0006-3495(86)83662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]



