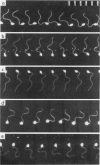
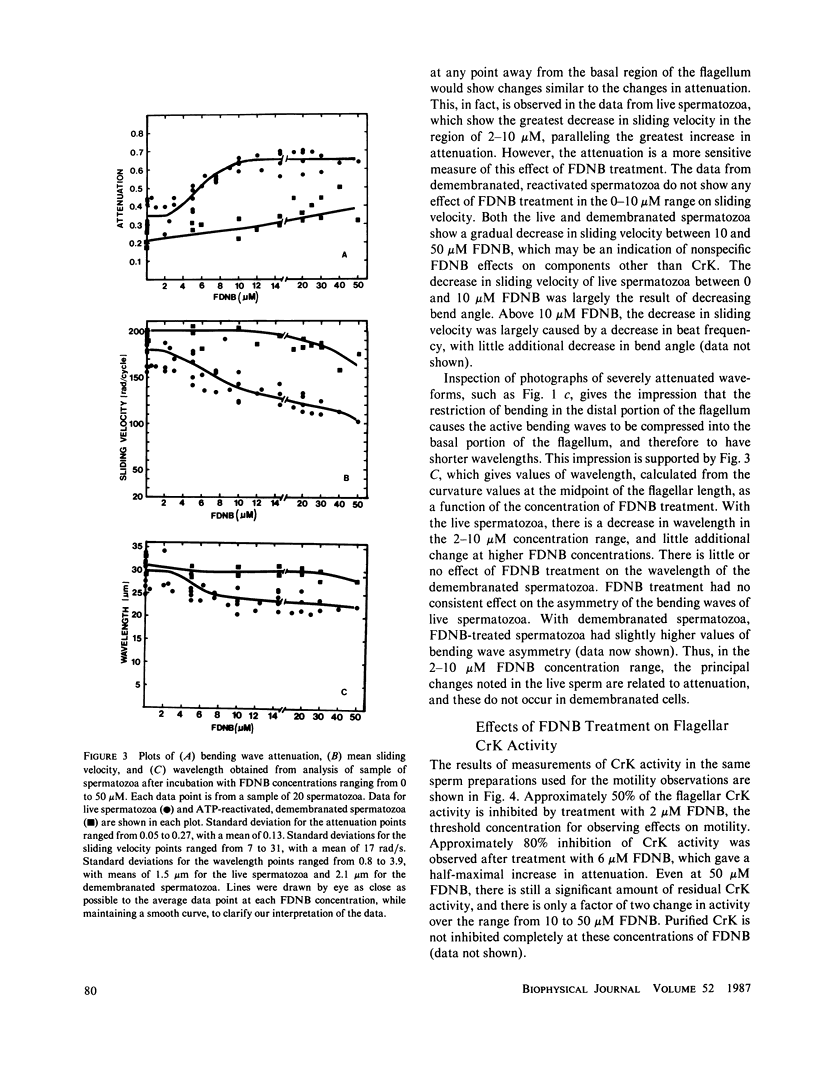
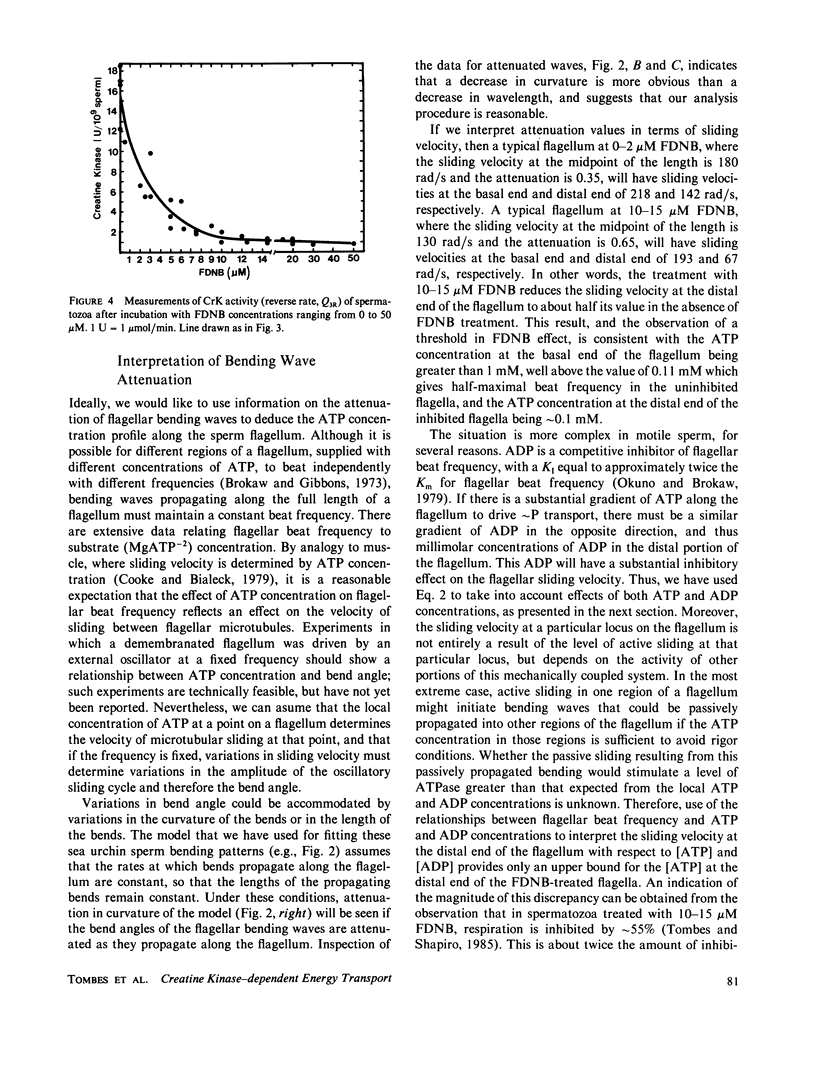
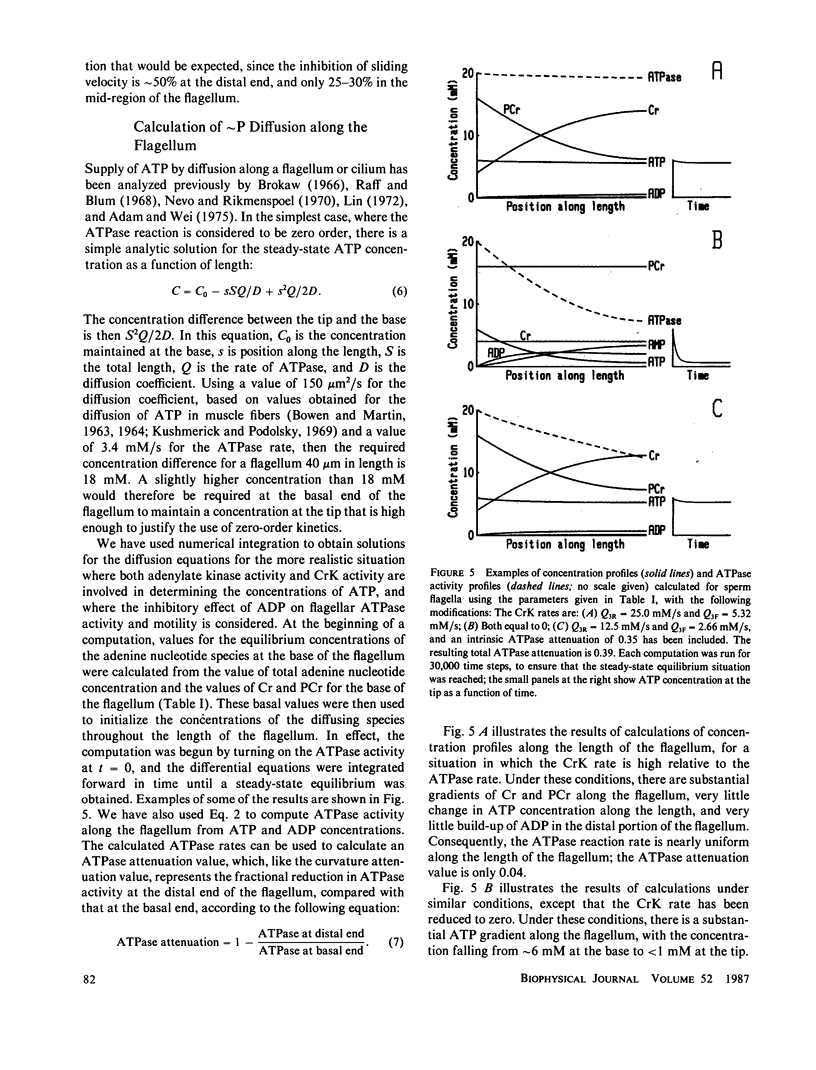
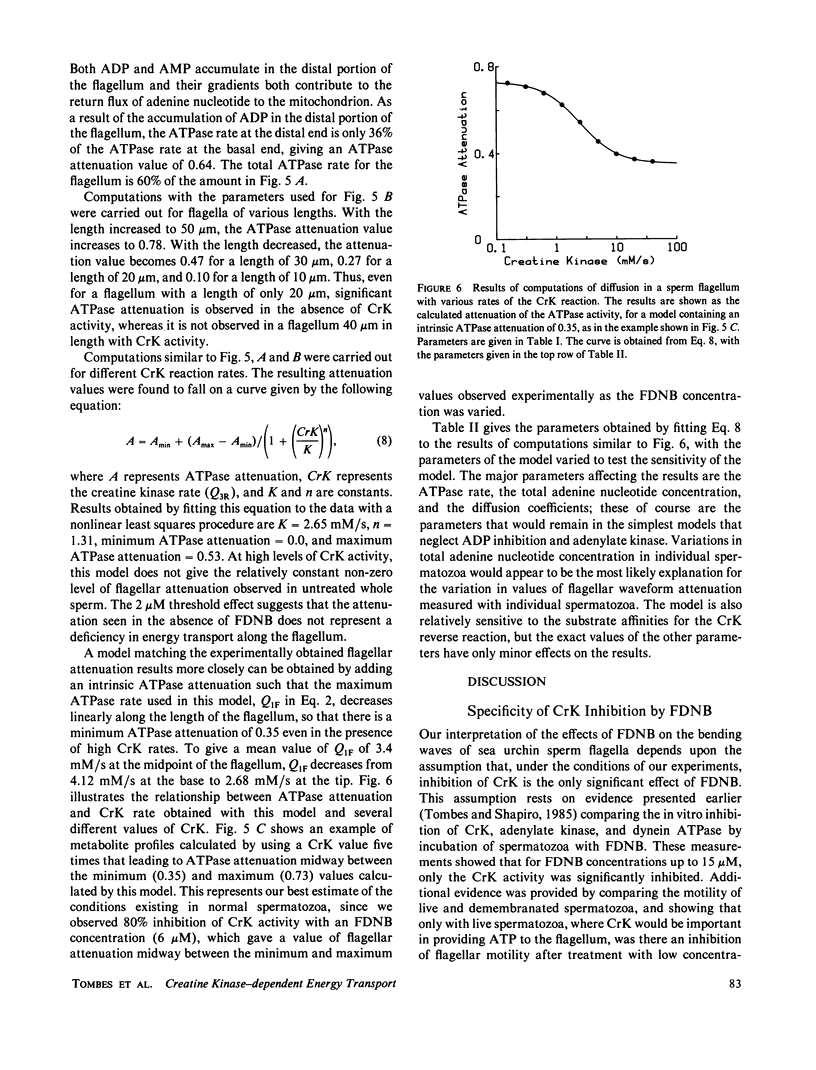
Abstract
The significance of a phosphocreatine (PCr) shuttle in the energy transport of motile spermatozoa (Tombes, R. M., and B. M. Shapiro, 1985, Cell, 41:325-334) has been tested by a quantitative analysis of motility. Computer-assisted analysis of stroboscopic photomicrographs of live sea urchin spermatozoa whose creatine kinase has been specifically inhibited by fluorodinitrobenzene reveals that motility is impaired due to a progressive damping of bending waves as they propagate along the flagellum. This lesion, which has been defined as attenuation and can be quantified, is repaired when these spermatozoa are demembranated and reactivated to swim with ATP. The implication that attenuation is due to the inhibition of energy transport via a PCr shuttle resulting in the decrease of ATP and accumulation of inhibitory levels of ADP distally has been supported by calculating sperm PCr and ATP levels resulting from diffusion along the flagellum. The specific alterations of motility seen with creatine kinase inhibition and their reversal with ATP are as expected from the model and provide strong support for the PCr shuttle in high energy phosphate transport.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam D. E., Wei J. Mass transport of ATP within the motile sperm. J Theor Biol. 1975 Jan;49(1):125–145. doi: 10.1016/s0022-5193(75)80023-3. [DOI] [PubMed] [Google Scholar]
- BOWEN W. J., MARTIN H. L. A STUDY OF DIFFUSION OF ATP THROUGH GLYCEROL-TREATED MUSCLE. Arch Biochem Biophys. 1963 Aug;102:286–292. doi: 10.1016/0003-9861(63)90182-6. [DOI] [PubMed] [Google Scholar]
- BOWEN W. J., MARTIN H. L. THE DIFFUSION OF ADENOSINE TRIPHOSPHATE THROUGH AQUEOUS SOLUTIONS. Arch Biochem Biophys. 1964 Jul;107:30–36. doi: 10.1016/0003-9861(64)90265-6. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Adenosine triphosphate usage by flagella. Science. 1967 Apr 7;156(3771):76–78. doi: 10.1126/science.156.3771.76. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Automated methods for estimation of sperm flagellar bending parameters. Cell Motil. 1984;4(6):417–430. doi: 10.1002/cm.970040603. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Computer simulation of flagellar movement. I. Demonstration of stable bend propagation and bend initiation by the sliding filament model. Biophys J. 1972 May;12(5):564–586. doi: 10.1016/S0006-3495(72)86104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw C. J. Computer simulation of flagellar movement. VI. Simple curvature-controlled models are incompletely specified. Biophys J. 1985 Oct;48(4):633–642. doi: 10.1016/S0006-3495(85)83819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw C. J. Cyclic AMP-dependent activation of sea urchin and tunicate sperm motility. Ann N Y Acad Sci. 1984;438:132–141. doi: 10.1111/j.1749-6632.1984.tb38282.x. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Effects of viscosity and ATP concentration on the movement of reactivated sea-urchin sperm flagella. J Exp Biol. 1975 Jun;62(3):701–719. doi: 10.1242/jeb.62.3.701. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J., Gibbons I. R. Localized activation of bending in proximal, medial and distal regions of sea-urchin sperm flagella. J Cell Sci. 1973 Jul;13(1):1–10. doi: 10.1242/jcs.13.1.1. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Mechanics and energetics of cilia. Am Rev Respir Dis. 1966 Mar;93(3 Suppl):32–40. doi: 10.1164/arrd.1966.93.3P2.32. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Non-sinusoidal bending waves of sperm flagella. J Exp Biol. 1965 Aug;43(1):155–169. doi: 10.1242/jeb.43.1.155. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Sperm motility. Methods Cell Biol. 1986;27:41–56. [PubMed] [Google Scholar]
- Christen R., Schackmann R. W., Dahlquist F. W., Shapiro B. M. 31P-NMR analysis of sea urchin sperm activation. Reversible formation of high energy phosphate compounds by changes in intracellular pH. Exp Cell Res. 1983 Nov;149(1):289–294. doi: 10.1016/0014-4827(83)90400-7. [DOI] [PubMed] [Google Scholar]
- Christen R., Schackmann R. W., Shapiro B. M. Elevation of the intracellular pH activates respiration and motility of sperm of the sea urchin, Strongylocentrotus purpuratus. J Biol Chem. 1982 Dec 25;257(24):14881–14890. [PubMed] [Google Scholar]
- Christen R., Schackmann R. W., Shapiro B. M. Metabolism of sea urchin sperm. Interrelationships between intracellular pH, ATPase activity, and mitochondrial respiration. J Biol Chem. 1983 May 10;258(9):5392–5399. [PubMed] [Google Scholar]
- Cooke R., Bialek W. Contraction of glycerinated muscle fibers as a function of the ATP concentration. Biophys J. 1979 Nov;28(2):241–258. doi: 10.1016/S0006-3495(79)85174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons B. H., Gibbons I. R. Flagellar movement and adenosine triphosphatase activity in sea urchin sperm extracted with triton X-100. J Cell Biol. 1972 Jul;54(1):75–97. doi: 10.1083/jcb.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons B. H., Tang W. J., Gibbons I. R. Organic anions stabilize the reactivated motility of sperm flagella and the latency of dynein 1 ATPase activity. J Cell Biol. 1985 Oct;101(4):1281–1287. doi: 10.1083/jcb.101.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I. R. Cilia and flagella of eukaryotes. J Cell Biol. 1981 Dec;91(3 Pt 2):107s–124s. doi: 10.1083/jcb.91.3.107s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansbrough J. R., Kopf G. S., Garbers D. L. The stimulation of sperm metabolism by a factor associated with eggs and by 8-bromo-guanosine 3',5'-monophosphate. Biochim Biophys Acta. 1980 Jun 5;630(1):82–91. doi: 10.1016/0304-4165(80)90139-7. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Sweeney H. L., Kushmerick M. J. A simple analysis of the "phosphocreatine shuttle". Am J Physiol. 1984 May;246(5 Pt 1):C365–C377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- Nevo A. C., Rikmenspoel R. Diffusion of ATP in sperm flagella. J Theor Biol. 1970 Jan;26(1):11–18. doi: 10.1016/s0022-5193(70)80027-3. [DOI] [PubMed] [Google Scholar]
- Okuno M., Brokaw C. J. Inhibition of movement of trition-demembranated sea-urchin sperm flagella by Mg2+, ATP4-, ADP and P1. J Cell Sci. 1979 Aug;38:105–123. doi: 10.1242/jcs.38.1.105. [DOI] [PubMed] [Google Scholar]
- Penningroth S. M., Cheung A., Olehnik K., Koslosky R. Mechanochemical coupling in the relaxation of rigor-wave sea urchin sperm flagella. J Cell Biol. 1982 Mar;92(3):733–741. doi: 10.1083/jcb.92.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff E. C., Blums J. J. A possible role for adenylate kinase in cilia: concentration profiles in a geometrically constrained dual enzyme system. J Theor Biol. 1968 Jan;18(1):53–71. doi: 10.1016/0022-5193(68)90170-7. [DOI] [PubMed] [Google Scholar]
- Schackmann R. W., Christen R., Shapiro B. M. Measurement of plasma membrane and mitochondrial potentials in sea urchin sperm. Changes upon activation and induction of the acrosome reaction. J Biol Chem. 1984 Nov 25;259(22):13914–13922. [PubMed] [Google Scholar]
- Tombes R. M., Shapiro B. M. Metabolite channeling: a phosphorylcreatine shuttle to mediate high energy phosphate transport between sperm mitochondrion and tail. Cell. 1985 May;41(1):325–334. doi: 10.1016/0092-8674(85)90085-6. [DOI] [PubMed] [Google Scholar]
- Warner F. D., Mitchell D. R. Dynein: the mechanochemical coupling adenosine triphosphatase of microtubule-based sliding filament mechanisms. Int Rev Cytol. 1980;66:1–43. doi: 10.1016/s0074-7696(08)61970-1. [DOI] [PubMed] [Google Scholar]