Abstract
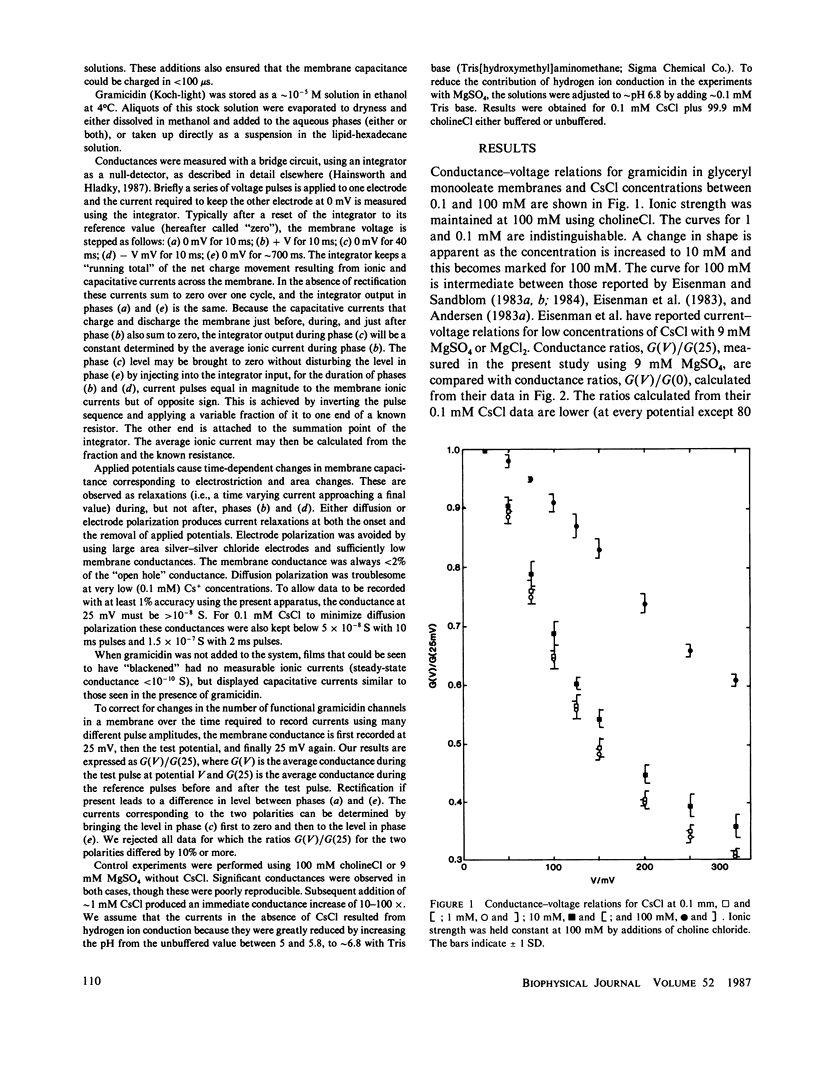
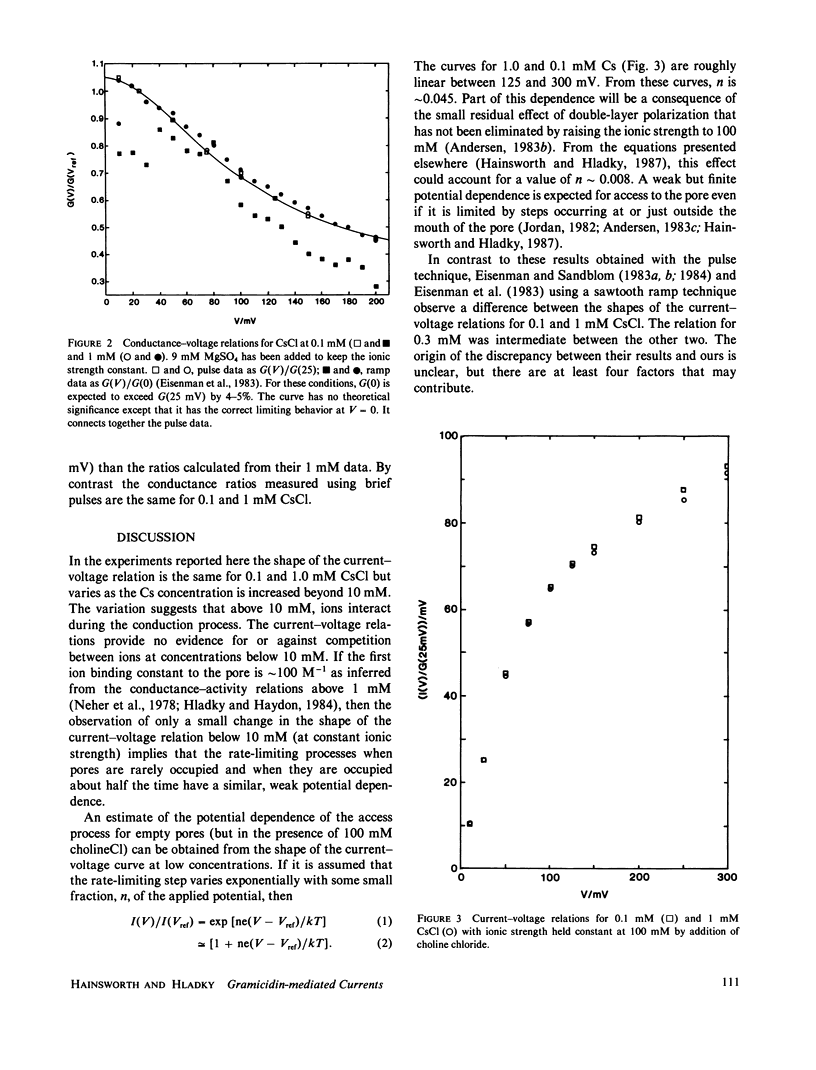
Current-voltage relations have been measured for the fluxes of caesium ions through pores formed by gramicidin in lipid bilayer membranes. The ionic currents have been separated from capacitative currents using a bridge circuit with an integrator as null-detector. The conductances during brief voltage pulses were small enough to avoid the effects of diffusion polarization and the ionic strength was raised using choline chloride or magnesium sulfate to reduce the effects of double-layer polarization. Under these conditions the current-voltage relations have the same shape at 0.1 and 1 mM, but different shapes for higher concentrations. These data demonstrate that the fluxes do not obey independence for concentrations above 10 mM, but they cannot be used in isolation to support a particular value of the binding constant. The shape observed at low concentrations suggests that entry of ions into the pore remains weakly potential dependent even at 300 mV.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S. Ion movement through gramicidin A channels. Interfacial polarization effects on single-channel current measurements. Biophys J. 1983 Feb;41(2):135–146. doi: 10.1016/S0006-3495(83)84415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S. Ion movement through gramicidin A channels. Single-channel measurements at very high potentials. Biophys J. 1983 Feb;41(2):119–133. doi: 10.1016/S0006-3495(83)84414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S. Ion movement through gramicidin A channels. Studies on the diffusion-controlled association step. Biophys J. 1983 Feb;41(2):147–165. doi: 10.1016/S0006-3495(83)84416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G., Hägglund J., Sandblom J., Enos B. The current-voltage behavior of ion channels: important features of the energy profile of the gramicidin channel deduced from the conductance-voltage characteristic in the limit of low ion concentration. Ups J Med Sci. 1980;85(3):247–257. doi: 10.3109/03009738009179195. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Sandblom J. P. Modeling the gramicidin channel: interpretation of experimental data using rate theory. Biophys J. 1984 Jan;45(1):88–90. doi: 10.1016/S0006-3495(84)84119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A., Andersen O. S. The gramicidin A channel: a review of its permeability characteristics with special reference to the single-file aspect of transport. J Membr Biol. 1981 Apr 30;59(3):155–171. doi: 10.1007/BF01875422. [DOI] [PubMed] [Google Scholar]
- Hainsworth A. H., Hladky S. B. Effects of double-layer polarization on ion transport. Biophys J. 1987 Jan;51(1):27–36. doi: 10.1016/S0006-3495(87)83308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim Biophys Acta. 1972 Aug 9;274(2):294–312. doi: 10.1016/0005-2736(72)90178-2. [DOI] [PubMed] [Google Scholar]
- Jordan P. C. Electrostatic modeling of ion pores. Energy barriers and electric field profiles. Biophys J. 1982 Aug;39(2):157–164. doi: 10.1016/S0006-3495(82)84503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sandblom J., Eisenman G. Ionic selectivity, saturation, and block in gramicidin A channels. II. Saturation behavior of single channel conductances and evidence for the existence of multiple binding sites in the channel. J Membr Biol. 1978 Apr 26;40(2):97–116. doi: 10.1007/BF01871143. [DOI] [PubMed] [Google Scholar]
- Neumcke B. Diffusion polarization at lipid bilayer membranes. Biophysik. 1971;7(2):95–105. doi: 10.1007/BF01190141. [DOI] [PubMed] [Google Scholar]
- Urban B. W., Hladky S. B., Haydon D. A. Ion movements in gramicidin pores. An example of single-file transport. Biochim Biophys Acta. 1980 Nov 4;602(2):331–354. doi: 10.1016/0005-2736(80)90316-8. [DOI] [PubMed] [Google Scholar]


