Abstract
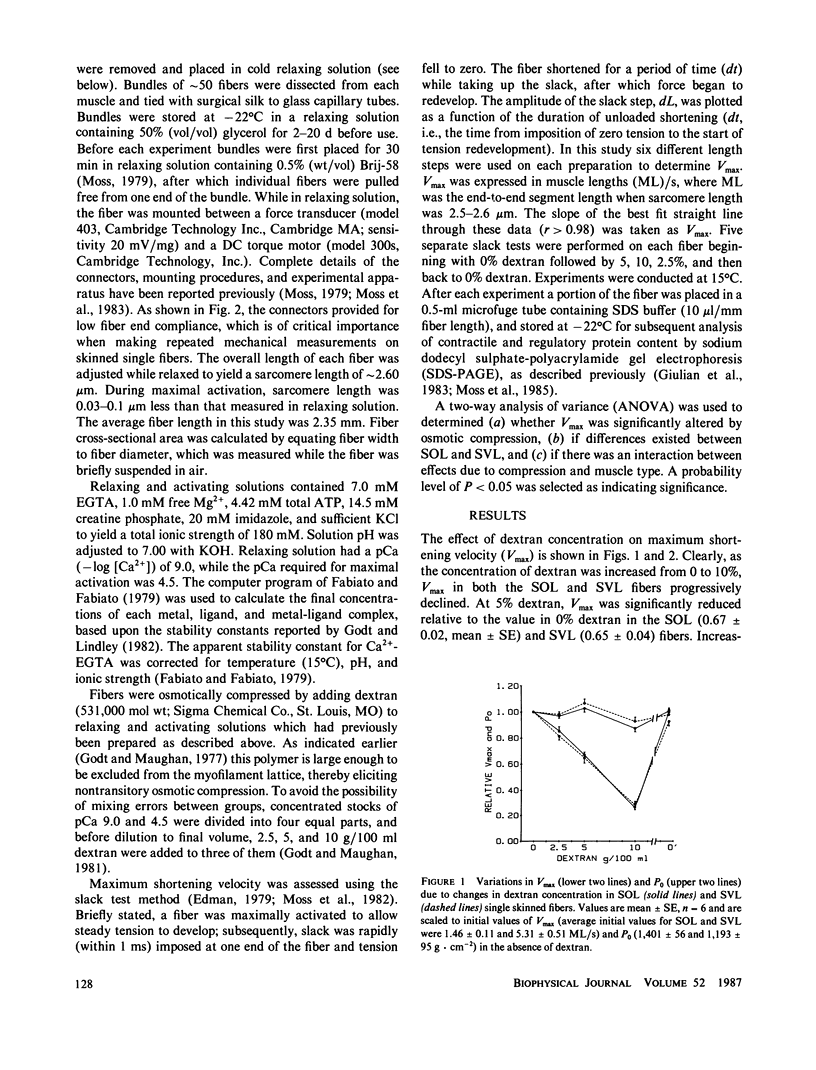
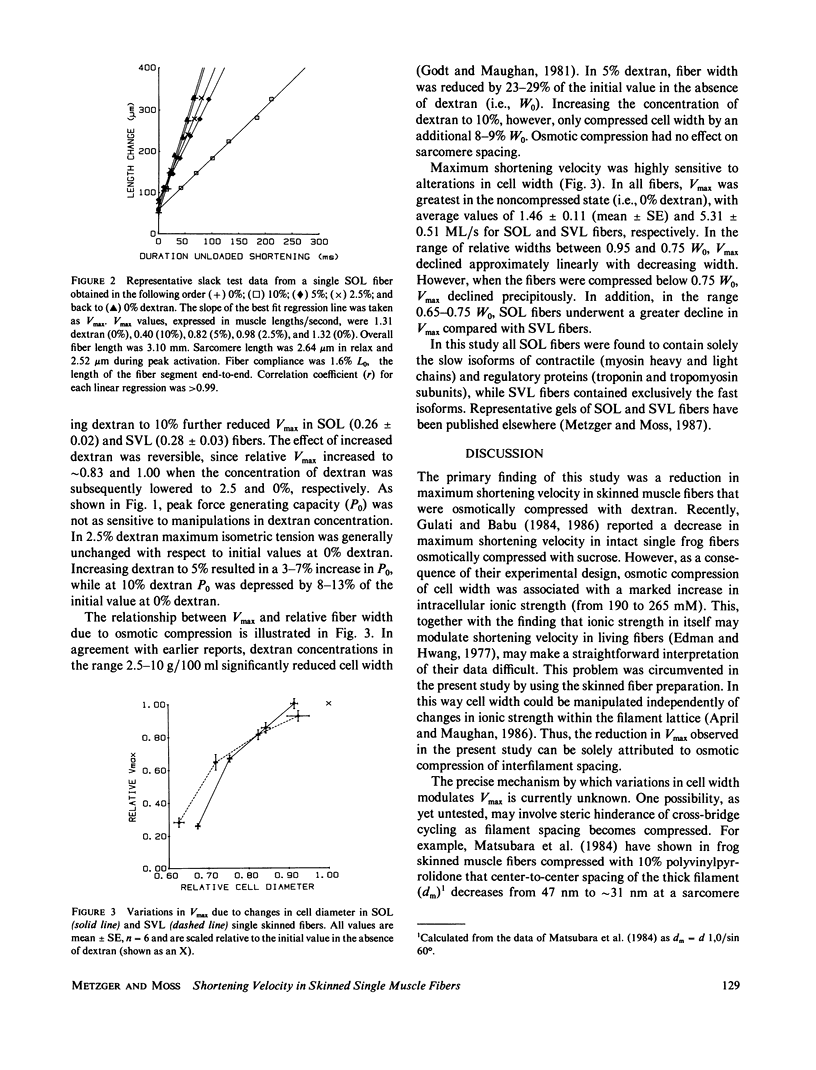
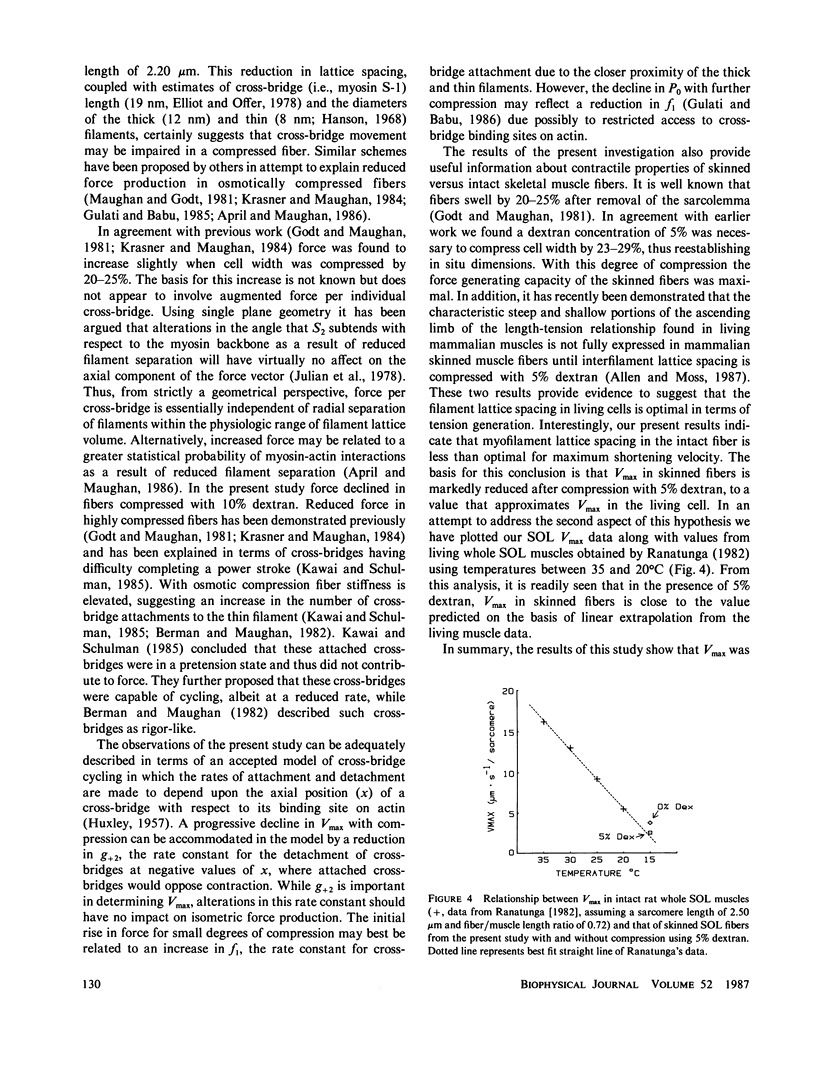
In this study maximum shortening velocity (Vmax) and isometric tension (P0) in skinned single fibers from rat slow soleus (SOL) and fast superficial vastus lateralis (SVL) muscles were examined after varying degrees of filament lattice compression with dextran. In both fiber types Vmax was greatest in the absence of dextran and decreased as the concentration of dextran was increased between 2.5 and 10 g/100 ml. At 10% dextran, which compressed fiber width by 31-38%, Vmax relative to the initial 0% dextran value was 0.28 +/- 0.03 (mean +/- SE) and 0.26 +/- 0.02 in SVL and SOL fibers, respectively. The effect of compression to depress Vmax was reversed completely by returning the fiber to 0% dextran. The force-generating capability of skinned fibers was not as sensitive to variations in cell width. In both the SOL and SVL fibers P0 increased by 3-7% when the concentration of dextran was increased from 0 to 5%. Further compression of lattice volume with 10% dextran resulted in a 8-13% decline in P0 relative to the initial value. While the precise mechanism by which filament lattice spacing modulates contractile function is not known, our results suggest that the major effect is upon the rate constant for cross-bridge detachment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- April E. W., Maughan D. W. Active force as a function of filament spacing in crayfish skinned muscle fibers. Pflugers Arch. 1986 Oct;407(4):456–460. doi: 10.1007/BF00652634. [DOI] [PubMed] [Google Scholar]
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Baldwin K. M., Klinkerfuss G. H., Terjung R. L., Molé P. A., Holloszy J. O. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol. 1972 Feb;222(2):373–378. doi: 10.1152/ajplegacy.1972.222.2.373. [DOI] [PubMed] [Google Scholar]
- Berman M. R., Maughan D. W. Axial elastic modulus as a function of relative fiber width in relaxed skinned skeletal muscle fibers. Pflugers Arch. 1982 Mar;393(1):99–103. doi: 10.1007/BF00582400. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Hwang J. C. The force-velocity relationship in vertebrate muscle fibres at varied tonicity of the extracellular medium. J Physiol. 1977 Jul;269(2):255–272. doi: 10.1113/jphysiol.1977.sp011901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A., Offer G. Shape and flexibility of the myosin molecule. J Mol Biol. 1978 Aug 25;123(4):505–519. doi: 10.1016/0022-2836(78)90204-8. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983 Mar;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. Influence of osmotic compression on calcium activation and tension in skinned muscle fibers of the rabbit. Pflugers Arch. 1981 Oct;391(4):334–337. doi: 10.1007/BF00581519. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. Swelling of skinned muscle fibers of the frog. Experimental observations. Biophys J. 1977 Aug;19(2):103–116. doi: 10.1016/S0006-3495(77)85573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Babu A. Critical dependence of calcium-activated force on width in highly compressed skinned fibers of the frog. Biophys J. 1985 Nov;48(5):781–787. doi: 10.1016/S0006-3495(85)83836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Babu A. Intrinsic shortening speed of temperature-jump-activated intact muscle fibers. Effects of varying osmotic pressure with sucrose and KCl. Biophys J. 1984 Feb;45(2):431–445. doi: 10.1016/S0006-3495(84)84166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Babu A. Kinetics of force redevelopment in isolated intact frog fibers in solutions of varied osmolarity. Biophys J. 1986 Apr;49(4):949–955. doi: 10.1016/S0006-3495(86)83723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hanson J. Recent x-ray diffraction studies of muscle. Q Rev Biophys. 1968 Jun;1(2):177–216. doi: 10.1017/s0033583500000536. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L., Sollins M. R. The mechanism for vertebrate striated muscle contraction. Circ Res. 1978 Jan;42(1):2–14. doi: 10.1161/01.res.42.1.2. [DOI] [PubMed] [Google Scholar]
- Kawai M., Schulman M. I. Crossbridge kinetics in chemically skinned rabbit psoas fibres when the actin-myosin lattice spacing is altered by dextran T-500. J Muscle Res Cell Motil. 1985 Jun;6(3):313–332. doi: 10.1007/BF00713172. [DOI] [PubMed] [Google Scholar]
- Krasner B., Maughan D. The relationship between ATP hydrolysis and active force in compressed and swollen skinned muscle fibers of the rabbit. Pflugers Arch. 1984 Feb;400(2):160–165. doi: 10.1007/BF00585033. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Goldman Y. E., Simmons R. M. Changes in the lateral filament spacing of skinned muscle fibres when cross-bridges attach. J Mol Biol. 1984 Feb 15;173(1):15–33. doi: 10.1016/0022-2836(84)90401-7. [DOI] [PubMed] [Google Scholar]
- Maughan D. W., Godt R. E. Inhibition of force production in compressed skinned muscle fibers of the frog. Pflugers Arch. 1981 May;390(2):161–163. doi: 10.1007/BF00590200. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. Effects of EDTA treatment upon the protein subunit composition and mechanical properties of mammalian single skeletal muscle fibers. J Cell Biol. 1983 Apr;96(4):970–978. doi: 10.1083/jcb.96.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. Physiological effects accompanying the removal of myosin LC2 from skinned skeletal muscle fibers. J Biol Chem. 1982 Aug 10;257(15):8588–8591. [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. The effects of partial extraction of TnC upon the tension-pCa relationship in rabbit skinned skeletal muscle fibers. J Gen Physiol. 1985 Oct;86(4):585–600. doi: 10.1085/jgp.86.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979 Jul;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga K. W. Temperature-dependence of shortening velocity and rate of isometric tension development in rat skeletal muscle. J Physiol. 1982 Aug;329:465–483. doi: 10.1113/jphysiol.1982.sp014314. [DOI] [PMC free article] [PubMed] [Google Scholar]


