Abstract
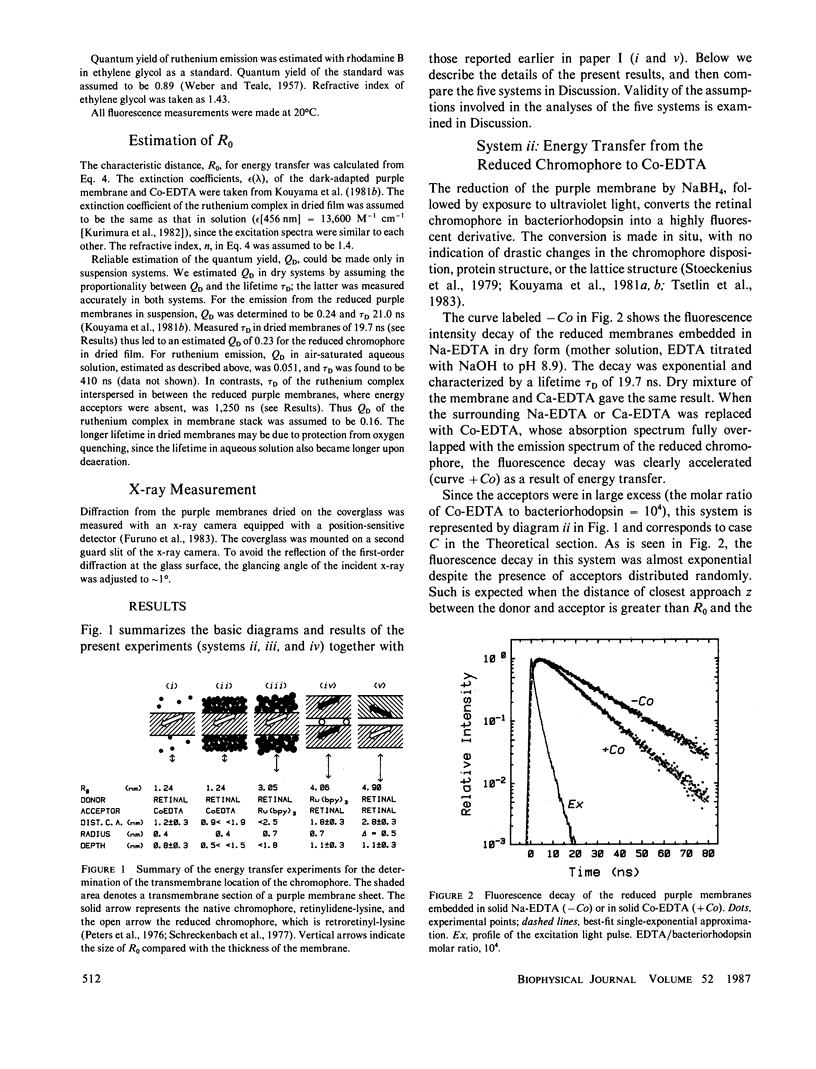
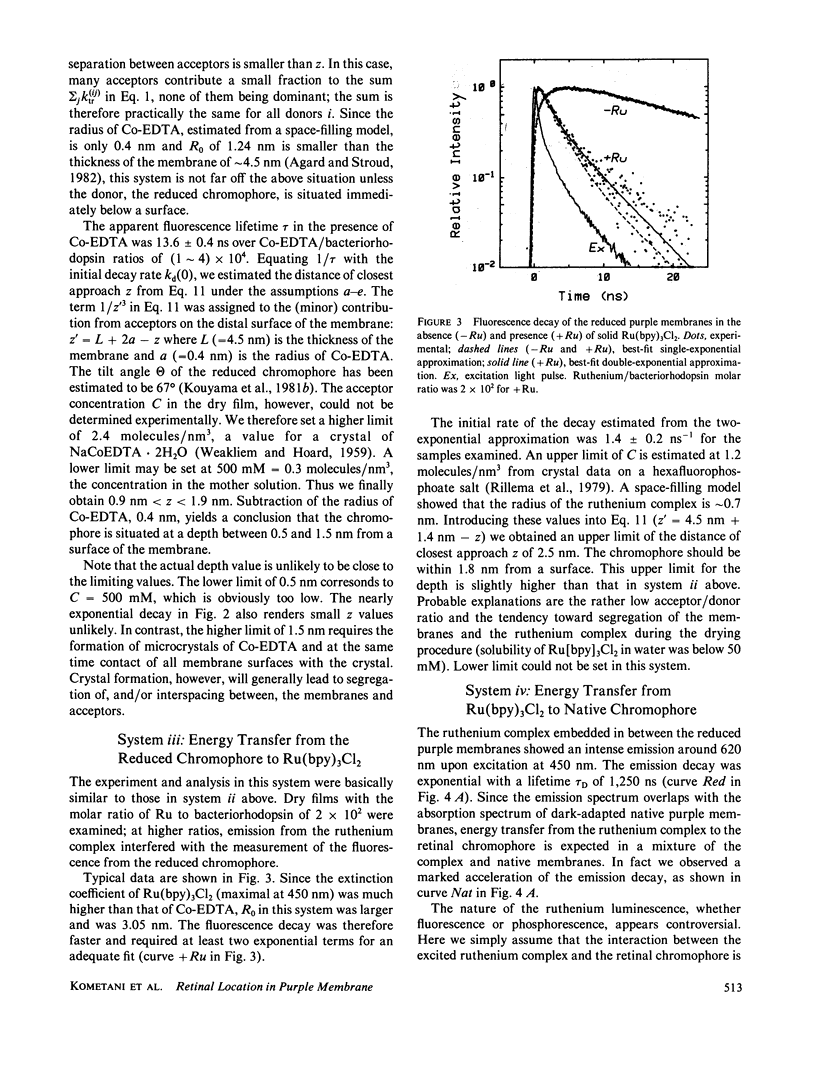
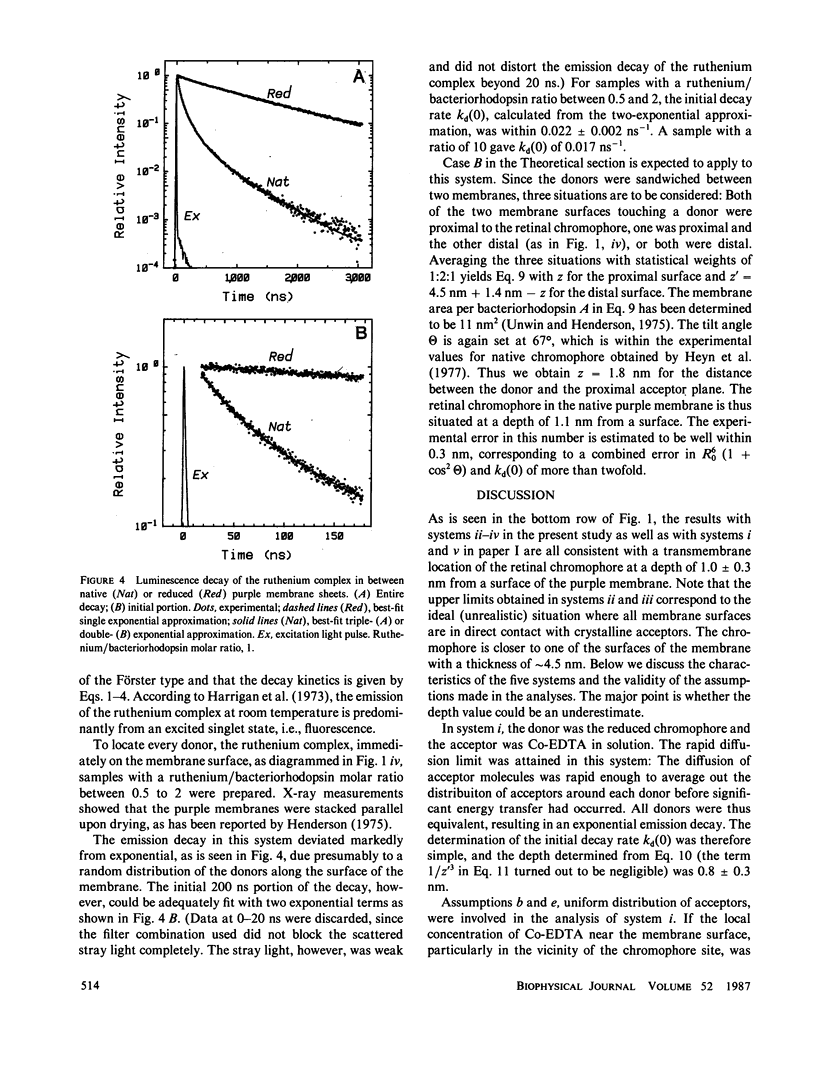
Transmembrane location of the retinal chromophore in the purple membrane of Halobacterium halobium was investigated in three different systems in which excitation energy transfer between the chromophore and external dye molecules condensed on the membrane surfaces was observed. In system ii, the energy donor was the retinal chromophore converted in situ to a fluorescent derivative. The fluorescent membranes were embedded in solid cobalt-EDTA, which served as energy acceptors. System iii was similar to system ii, except that the acceptors were tris(2,2′-bipyridyl)ruthenium(II) complex in solid form. The positively charged ruthenium complex had a radius of 0.7 nm, whereas the cobalt complex in system ii was smaller (radius ∼0.4 nm) and negatively charged. System iv was stacked sheets of native purple membrane with interspersed ruthenium complex; energy transfer from the luminescent ruthenuim complex to the native retinal chromophore was observed. The energy transfer rates in these three systems, and in two additional systems already described (Kouyama, T., K. Kinosita, Jr., and A. Ikegami, 1983, J. Mol. Biol., 165:91-107), were all consistent with a location of the retinal chromophore at a depth of 1.0 ± 0.3 nm from a surface of the purple membrane. All the analyses in the present work involved an assumption that contacts between the external dye molecules and membrane surfaces were maximal; the depth values obtained cannot be underestimates. The chromophore therefore must be outside the middle one-third of the thickness, ∼4.5 nm, of the purple membrane.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A., Stroud R. M. Linking regions between helices in bacteriorhodopsin revealed. Biophys J. 1982 Mar;37(3):589–602. [PMC free article] [PubMed] [Google Scholar]
- Bayley H., Huang K. S., Radhakrishnan R., Ross A. H., Takagaki Y., Khorana H. G. Site of attachment of retinal in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2225–2229. doi: 10.1073/pnas.78.4.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Braiman M., Mathies R. Resonance Raman spectra of bacteriorhodopsin's primary photoproduct: evidence for a distorted 13-cis retinal chromophore. Proc Natl Acad Sci U S A. 1982 Jan;79(2):403–407. doi: 10.1073/pnas.79.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno T., Ikegami A., Kihara H., Yoshida M., Kagawa Y. Small-angle X-ray scattering study of adenosine triphosphatase from thermophilic bacterium PS3. J Mol Biol. 1983 Oct 15;170(1):137–153. doi: 10.1016/s0022-2836(83)80230-7. [DOI] [PubMed] [Google Scholar]
- Hayward S. B., Stroud R. M. Projected structure of purple membrane determined to 3.7 A resolution by low temperature electron microscopy. J Mol Biol. 1981 Sep 25;151(3):491–517. doi: 10.1016/0022-2836(81)90007-3. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Müller U. Transient and linear dichroism studies on bacteriorhodopsin: determination of the orientation of the 568 nm all-trans retinal chromophore. J Mol Biol. 1977 Dec 15;117(3):607–620. doi: 10.1016/0022-2836(77)90060-2. [DOI] [PubMed] [Google Scholar]
- Huang K. S., Radhakrishnan R., Bayley H., Khorana H. G. Orientation of retinal in bacteriorhodopsin as studied by cross-linking using a photosensitive analog of retinal. J Biol Chem. 1982 Nov 25;257(22):13616–13623. [PubMed] [Google Scholar]
- Jubb J. S., Worcester D. L., Crespi H. L., Zaccaï G. Retinal location in purple membrane of Halobacterium halobium: a neutron diffraction study of membranes labelled in vivo with deuterated retinal. EMBO J. 1984 Jul;3(7):1455–1461. doi: 10.1002/j.1460-2075.1984.tb01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katre N. V., Wolber P. K., Stoeckenius W., Stroud R. M. Attachment site(s) of retinal in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4068–4072. doi: 10.1073/pnas.78.7.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana H. G., Gerber G. E., Herlihy W. C., Gray C. P., Anderegg R. J., Nihei K., Biemann K. Amino acid sequence of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5046–5050. doi: 10.1073/pnas.76.10.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. I., Stoekenius W., Crespi H. L., Schoenborn B. P. The location of retinal in the purple membrane profile by neutron diffraction. J Mol Biol. 1979 Jun 5;130(4):395–404. doi: 10.1016/0022-2836(79)90430-3. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kataoka R., Kimura Y., Gotoh O., Ikegami A. Dynamic structure of biological membranes as probed by 1,6-diphenyl-1,3,5-hexatriene: a nanosecond fluorescence depolarization study. Biochemistry. 1981 Jul 21;20(15):4270–4277. doi: 10.1021/bi00518a006. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Kimura Y., Kinosita K., Jr, Ikegami A. Location and orientation of the chromophore in bacteriorhodopsin. Analysis by fluorescence energy transfer. J Mol Biol. 1981 Dec 5;153(2):337–359. doi: 10.1016/0022-2836(81)90282-5. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Kinosita K., Jr, Ikegami A. Fluorescence energy transfer studies of transmembrane location of retinal in purple membrane. J Mol Biol. 1983 Mar 25;165(1):91–107. doi: 10.1016/s0022-2836(83)80244-7. [DOI] [PubMed] [Google Scholar]
- Lemke H. D., Oesterhelt D. Lysine 216 is a binding site of the retinyl moiety in bacteriorhodopsin. FEBS Lett. 1981 Jun 15;128(2):255–260. doi: 10.1016/0014-5793(81)80093-2. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E., Johnson A. H., Akhtar M. The identification of Lys216 as the retinal binding residue in bacteriorhodopsin. FEBS Lett. 1981 Aug 3;130(2):187–193. doi: 10.1016/0014-5793(81)81116-7. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Abdulaev N. G., Feigina M. Y., Kiselev A. V., Lobanov N. A. The structural basis of the functioning of bacteriorhodopsin: an overview. FEBS Lett. 1979 Apr 15;100(2):219–224. doi: 10.1016/0014-5793(79)80338-5. [DOI] [PubMed] [Google Scholar]
- Peters J., Peters R., Stoeckenius W. A photosensitive product of sodium borohydride reduction of bacteriorhodopsin. FEBS Lett. 1976 Jan 15;61(2):128–134. doi: 10.1016/0014-5793(76)81019-8. [DOI] [PubMed] [Google Scholar]
- Pettei M. J., Yudd A. P., Nakanishi K., Henselman R., Stoeckenius W. Identification of retinal isomers isolated from bacteriorhodopsin. Biochemistry. 1977 May 3;16(9):1955–1959. doi: 10.1021/bi00628a031. [DOI] [PubMed] [Google Scholar]
- Schreckenbach T., Walckhoff B., Oesterhelt D. Studies on the retinal-protein interaction in bacteriorhodopsin. Eur J Biochem. 1977 Jun 15;76(2):499–511. doi: 10.1111/j.1432-1033.1977.tb11620.x. [DOI] [PubMed] [Google Scholar]
- Seiff F., Wallat I., Ermann P., Heyn M. P. A neutron diffraction study on the location of the polyene chain of retinal in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 May;82(10):3227–3231. doi: 10.1073/pnas.82.10.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W. The rhodopsin-like pigments of halobacteria: light-energy and signal transducers in an archaebacterium. Trends Biochem Sci. 1985 Dec;10(12):483–486. doi: 10.1016/0968-0004(85)90210-5. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Terner J., Hsieh C. L., Burns A. R., El-Sayed M. A. Time-resolved resonance Raman spectroscopy of intermediates of bacteriorhodopsin: The bK(590) intermediate. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3046–3050. doi: 10.1073/pnas.76.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Carlsen W. F., Stryer L. Fluorescence energy transfer in the rapid-diffusion limit. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5746–5750. doi: 10.1073/pnas.75.12.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Glaccum M., Nelson B., Ebrey T. G. Light isomerizes the chromophore of bacteriorhodopsin. Nature. 1980 Sep 25;287(5780):351–353. doi: 10.1038/287351a0. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Zaccai G., Gilmore D. J. Areas of hydration in the purple membrane of Halobacterium halobium: a neutron diffraction study. J Mol Biol. 1979 Aug 5;132(2):181–191. doi: 10.1016/0022-2836(79)90390-5. [DOI] [PubMed] [Google Scholar]


