Abstract
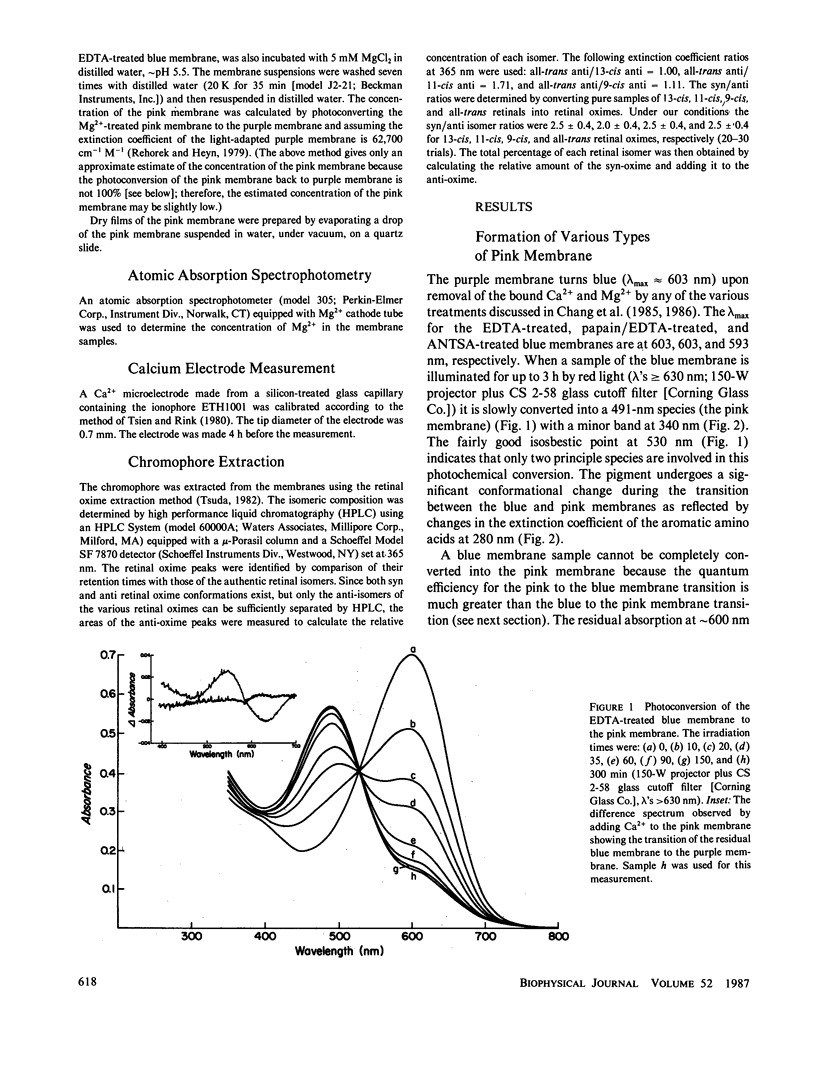
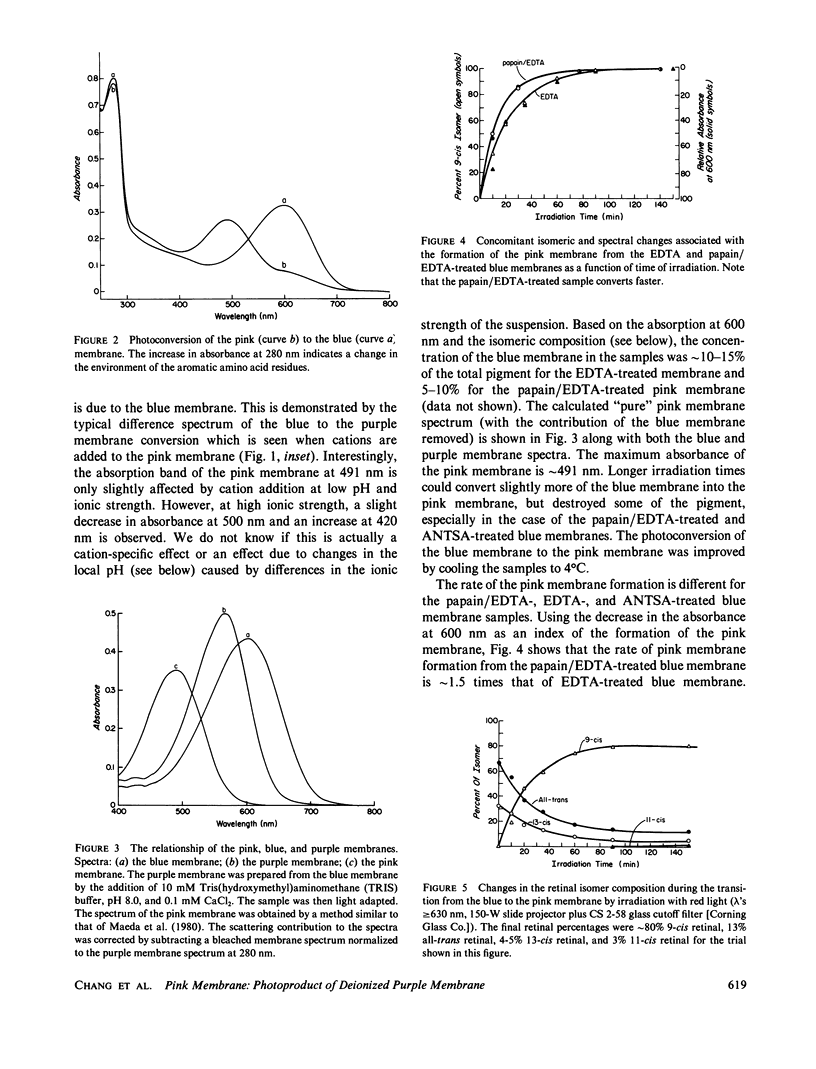
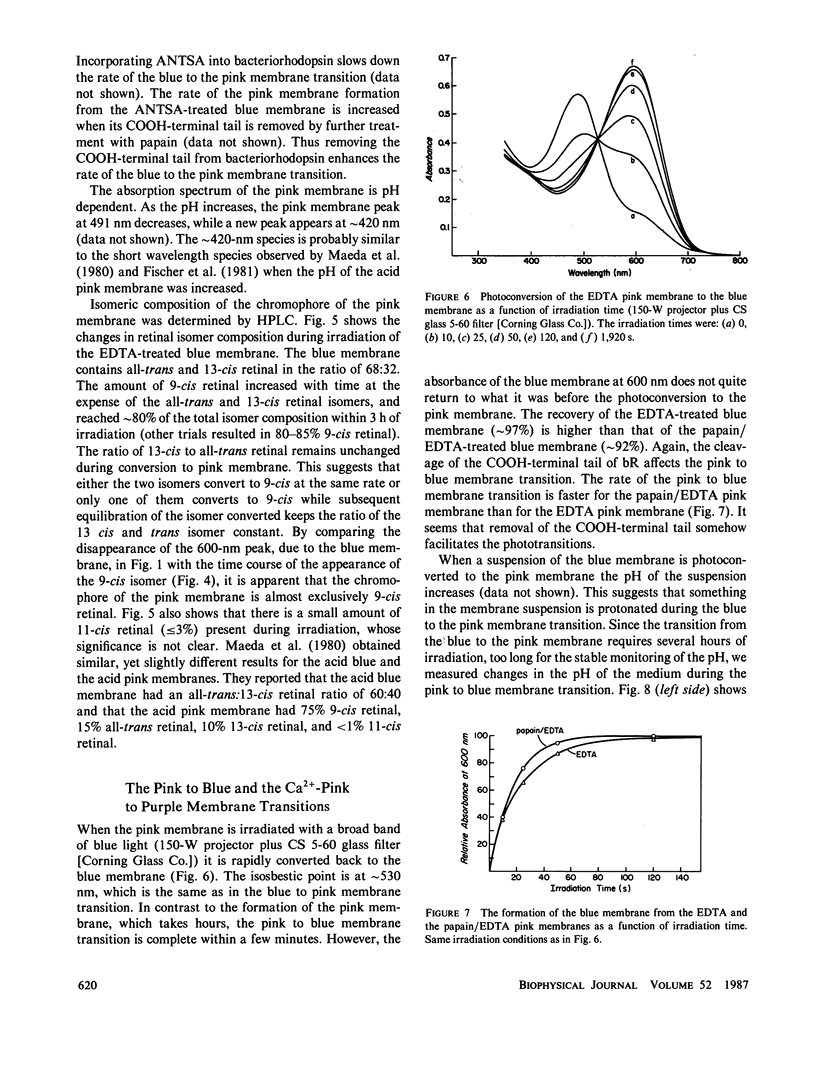
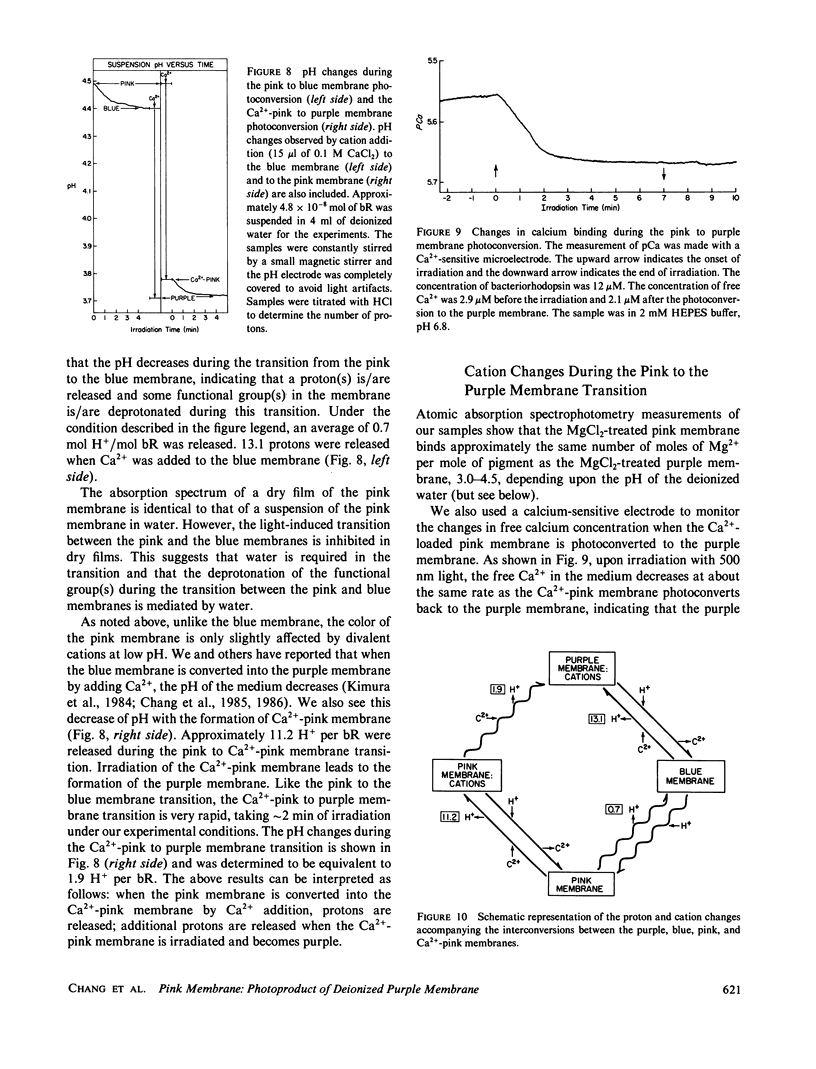
When cations are removed from the purple membrane of Halobacterium halobium it turns blue (λmax = 603 nm); continuous irradiation with intense red light (λ's ≥ 630 nm) converts this deionized blue membrane into a pink membrane (λmax ≈ 491 nm). The rate and extent of the transformation from the blue to the pink membrane is facilitated by the removal of the last twenty COOH-terminal amino acids of bacteriorhodopsin. While the chromophore of the blue membrane is a 32:68 mixture of the 13-cis and all-trans isomers of retinal, the chromophore of the pink membrane is 9-cis rectinal. The quantum efficiency of the pink to blue membrane photoconversion is relatively high compared with that of the blue to pink membrane photoconversion. Proton release is observed when the pink membrane is converted to the blue form, and proton uptake occurs during the reverse transition. Unlike the blue membrane, the absorbance maximum of the pink membrane is only slightly affected by cation addition at low pH and ionic strength.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Chen J. G., Govindjee R., Ebrey T. Cation binding by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 Jan;82(2):396–400. doi: 10.1073/pnas.82.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Jonas R., Melchiore S., Govindjee R., Ebrey T. G. Mechanism and role of divalent cation binding of bacteriorhodopsin. Biophys J. 1986 Mar;49(3):731–739. doi: 10.1016/S0006-3495(86)83699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee R., Ohno K., Chang C. H., Ebrey T. G. The C-terminal tail of bacteriorhodopsin--its conformation and role in proton pumping. Prog Clin Biol Res. 1984;164:13–25. [PubMed] [Google Scholar]
- Govindjee R., Ohno K., Ebrey T. G. Effect of the removal of the COOH-terminal region of bacteriorhodopsin on its light-induced H+ changes. Biophys J. 1982 Apr;38(1):85–87. doi: 10.1016/S0006-3495(82)84534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Ikegami A., Stoeckenius W. Salt and pH-dependent changes of the purple membrane absorption spectrum. Photochem Photobiol. 1984 Nov;40(5):641–646. doi: 10.1111/j.1751-1097.1984.tb05353.x. [DOI] [PubMed] [Google Scholar]
- Liao M. J., Khorana H. G. Removal of the carboxyl-terminal peptide does not affect refolding or function of bacteriorhodopsin as a light-dependent proton pump. J Biol Chem. 1984 Apr 10;259(7):4194–4199. [PubMed] [Google Scholar]
- Maeda A., Iwasa T., Yoshizawa T. Formation of 9-cis- and 11-cis-retinal pigments from bacteriorhodopsin by irradiating purple membrane in acid. Biochemistry. 1980 Aug 5;19(16):3825–3831. doi: 10.1021/bi00557a027. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Pande C., Callender R. H., Chang C. H., Ebrey T. G. Resonance Raman study of the pink membrane photochemically prepared from the deionized blue membrane of H. halobium. Biophys J. 1986 Sep;50(3):545–549. doi: 10.1016/S0006-3495(86)83493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehorek M., Heyn M. P. Binding of all-trans-retinal to the purple membrane. Evidence for cooperativity and determination of the extinction coefficient. Biochemistry. 1979 Oct 30;18(22):4977–4983. doi: 10.1021/bi00589a027. [DOI] [PubMed] [Google Scholar]
- Renthal R., Dawson N., Tuley J., Horowitz P. Constraints on the flexibility of bacteriorhodopsin's carboxyl-terminal tail at the purple membrane surface. Biochemistry. 1983 Jan 4;22(1):5–12. doi: 10.1021/bi00270a601. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Wallace B. A., Henderson R. Location of the carboxyl terminus of bacteriorhodopsin in purple membrane. Biophys J. 1982 Sep;39(3):233–239. doi: 10.1016/S0006-3495(82)84513-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


