Abstract
The murine d-galactosamine (d-gal) model of tumor necrosis factor alpha (TNF-α) hypersensitization was used as an initial tool to investigate the potential contribution of TNF-α to lethal intraperitoneal (i.p.) infection with Enterococcus faecalis. d-gal sensitized mice to lethal E. faecalis infection, whereas dexamethasone and neutralizing anti-TNF-α antibody protected d-gal-treated, E. faecalis-infected mice, implicating TNF-α in the lethal response to E. faecalis infection in d-gal-treated mice. Circulating TNF-α was undetectable for at least 8 h following i.p. E. faecalis infection, although low peritoneal levels of TNF-α were detected within 3 h, suggesting that localized TNF-α production contributed to the lethal response to E. faecalis infection in d-gal-treated mice. Although i.p. E. faecalis infection failed to induce a detectable systemic TNF-α response, circulating Interleukin-6 (IL-6) was detected within 3 h of infection. IL-6 was also detected in the peritoneum within an hour of infection, prior to the appearance of peritoneal TNF-α. In striking contrast to in vivo results, E. faecalis induced a potent and rapid TNF-α response from both mouse peritoneal macrophages and the RAW 264.7 cell line in vitro. This led us to hypothesize that TNF-α production in response to E. faecalis infection is suppressed by IL-6 in vivo. In vitro experiments demonstrated a statistically significant, but modest, inhibitory effect of IL-6 on TNF-α production by RAW cells stimulated with E. faecalis. Collectively, these data indicate that acute, lethal E. faecalis infection appears to induce an unusual cytokine response that differs in character from that previously described for most other gram-positive and gram-negative bacteria.
Recent estimates indicate that between 300,000 and 500,000 Americans are diagnosed with sepsis annually and that the mortality associated with this condition remains between 20 and 50% despite significant advances in antimicrobial and supportive therapy (48). The pathogenesis of sepsis is recognized to involve the systemic production of a diverse array of inflammatory cytokines by several host cell types (e.g., monocytes-macrophages, endothelial cells, and neutrophils) in response to microbes or microbial products (8, 20). This inflammatory cascade can become self-sustaining when cytokines produced early in the infectious process (e.g., tumor necrosis factor alpha [TNF-α] and interleukin-1 [IL-1]) induce further production of these and other proinflammatory cytokines (20, 44)
Agents directed at common triggers for the sepsis syndrome (e.g., lipopolysaccharide [LPS]) or cytokines (e.g., TNF-α and IL-1) associated with systemic inflammation would, at least conceptually, be attractive therapeutic targets (1, 14). Regrettably, most of these potentially novel therapeutic approaches have failed to significantly affect the overall mortality of sepsis patients despite their success in many experimental animal models of sepsis (1, 2, 9, 16, 29, 38). One potential explanation for these repeated clinical failures is that sepsis, in fact, represents a heterogeneous collection of clinically related diseases whose pathogenesis may vary substantially, depending upon the microbe responsible for inducing the systemic proinflammatory cascade (e.g., gram-negative versus gram-positive organisms) (6, 34, 43).
The potential significance of differences in the host inflammatory response to gram-positive versus gram-negative bacterial infections was strongly suggested by a recent phase II clinical trial evaluating the therapeutic efficacy of soluble type II (p75) TNF-α receptor-Fc fusion protein constructs in reducing sepsis-related mortality (16). Although no overall survival benefit was observed in septic patients enrolled in that study, one subgroup of patients manifested a statistically significant dose-dependent increase in 28-day mortality relative to that of placebo-treated patients (16). This deleterious effect of anti-TNF-α therapy was identified in patients with gram-positive sepsis and was not observed in patients with gram-negative sepsis (16). These findings support the concept that TNF-α may benefit the host under at least some conditions of gram-positive sepsis, despite the general consensus that TNF-α is among the most harmful endogenous substances produced during sepsis. It is also worth noting that mice that are pretreated with neutralizing anti-TNF-α antibody or that are genetically deficient for the 55-kDa receptor for TNF-α show increased mortality when experimentally infected with the gram-positive bacterium Listeria monocytogenes (31, 35). In view of the significant recent increase in the incidence of nosocomial infections and sepsis attributable to gram-positive bacteria, more detailed evaluation of microbe-specific differences in the pathogenesis of sepsis appears warranted (7, 34). Findings from such studies have the potential to increase opportunities for new therapeutic approaches to the treatment of sepsis.
The murine d-galactosamine (d-gal) sensitization infection model has been extensively utilized to investigate the host inflammatory response in septic shock (18, 41, 42, 49). In this model, intraperitoneal (i.p.) administration of d-gal, which reversibly inhibits hepatocyte protein synthesis for approximately 2 to 4 h, also markedly sensitizes mice to the lethal effects of both endotoxin and gram-negative bacteria (11, 15). Experimental evidence strongly supports the conclusion that lethality in the d-gal model is, in large part, caused by TNF-α produced by host inflammatory cells in response to endotoxin (18, 19, 28). In this respect, pretreatment of mice with either neutralizing anti-TNF-α antibody or dexamethasone (DEX), which, among other effects, inhibits TNF-α synthesis, has been shown to reverse d-gal sensitization to endotoxin (18, 28, 41, 42). Additionally, d-gal treatment only minimally alters sensitivity to endotoxin in mice that are genetically deficient in either TNF-α or the 55-kDa receptor for TNF-α (33). Lastly, d-gal-treated mice are also rendered hypersensitive to the lethal effects of TNF-α by itself (19). Thus, the d-gal mouse model has been generally accepted as a useful model with which to assess the potential deleterious effects of TNF-α.
We recently reported marked differences in the pathogenic mechanisms and contributory roles of TNF-α in lethality induced by Staphylococcus aureus and Escherichia coli in the d-gal model (41). Pretreatment of mice with d-gal reduced the lethal dose of E. coli by approximately 4 orders of magnitude. Importantly, the protective effects of DEX treatment on d-gal-sensitized mice challenged with E. coli were about 150-fold. Collectively, these observations suggest that E. coli-mediated lethality occurred primarily via TNF-α-dependent mechanisms. In contrast, when mice were infected with S. aureus, d-gal pretreatment had essentially no differential impact on lethality (<10-fold) and DEX pretreatment failed to provide any significant protection of d-gal-treated mice. The latter data suggested only a minimal role for TNF-α as a lethal mediator in S. aureus infection in the experimental d-gal model. In vitro studies supported these findings; E. coli induced high levels TNF-α production in cultures of mouse peritoneal macrophages relative to those induced by S. aureus (an approximately 100-fold difference). Collectively, these observations support the conclusion that TNF-α plays a critical role in lethality induced by an endotoxin-containing gram-negative organism (e.g., E. coli) in d-gal-treated mice but contributes little to lethality induced by S. aureus, a gram-positive organism lacking endotoxin (41).
In this report, we describe the results of studies designed to investigate the role of TNF-α in lethality induced by the clinically important gram-positive bacterium Enterococcus faecalis with the same d-gal model described above. Enterococcus spp. are among the most common causes of gram-positive sepsis, and these infections may be extremely difficult to treat because of the organism's broad resistance to many antimicrobial agents (23, 27). Mortality rates attributable to enterococcal bacteremia are similar to those seen in gram-negative bacteremia, yet the inflammatory cascade associated with enterococcal sepsis has not been well characterized (13, 22, 26). Elucidation of the role of TNF-α and other inflammatory cytokines would therefore be a crucial first step in understanding the pathogenesis of enterococcal sepsis and death. In this communication, we report that E. faecalis appears to involve TNF-α in the induction of mortality and, in this respect, behaves more like a gram-negative than a gram-positive bacterium in the experimental d-gal model. In support of this conclusion, neutralizing antibody to TNF-α provided protection to d-gal-treated mice challenged with lethal doses of E. faecalis. Interestingly, a systemic TNF-α response was essentially undetectable following infection with two clinical isolates of E. faecalis, suggesting that localized TNF-α production may be sufficient to trigger a lethal response in the d-gal model. In striking contrast to the results obtained with the d-gal model, our results also suggest that acute E. faecalis infection can result in death by a predominantly TNF-α-independent mechanism in mice not previously treated with d-gal. Additionally, a systemic IL-6 response to E. faecalis infection develops in the absence of circulating TNF-α and peritoneal IL-6 is detected prior to peritoneal TNF-α, suggesting that E. faecalis induces IL-6 by a predominantly TNF-α-independent mechanism. Collectively, these data establish a relatively unique cytokine profile in response to potentially lethal E. faecalis infections in mice.
MATERIALS AND METHODS
Bacterial isolates.
E. coli O111:B4 was obtained from List Biological Laboratories (Campbell, Calif.). S. aureus M (type 1) was a gift from Chia Y. Lee, Department of Microbiology, Molecular Genetics, and Immunology, University of Kansas Medical Center. Two E. faecalis isolates (CP-1 and CP-2) were clinical isolates from the collections of the Truman Medical Center (Kansas City, Mo.). All other bacteria were generous gifts from Rebecca Horvat and came from the collection of clinical isolates at the University of Kansas Medical Center (Kansas City). These included five S. aureus strains, one Streptococcus mitis strain, one Streptococcus pneumoniae strain, one E. faecalis strain (K9), two E. coli strains, four Klebsiella pneumoniae strains, one Citrobacter diversus strain, one Pseudomonas aeruginosa strain, and one Proteus mirabilis strain.
Several colonies from a streaked plate grown overnight on Trypticase soy agar were used to initiate bacterial growth. Bacteria were inoculated into Trypticase soy broth and grown overnight at 37°C with aeration. On the following morning, 0.1 ml of this culture was transferred to 50 ml of fresh medium and the bacteria were grown to mid-log phase. The bacteria were then washed three times in sterile saline and diluted in either cell culture medium (see below) for in vitro stimulation, sterile saline for in vivo cytokine stimulation, or phosphate-buffered saline (PBS) for sepsis lethality studies.
Experimental animals.
Outbred CF-1 female mice, 6 to 8 weeks of age, were purchased from Charles River Laboratories, Inc. (Wilmington, Mass.). All mice were monitored at either the University of Kansas Medical Center or the University of Missouri, Kansas City, animal facilities for 5 to 10 days before being used for experiments, with food and water provided ad libitum. Both animal facilities are American Association for Assessment and Accreditation of Laboratory Animal Care accredited.
Reagents.
Purified LPS from the deep rough mutant of E. coli D31m4 was a generous gift from Nilofer Qureshi (36). d-gal was obtained through Sigma Chemical Co. (St. Louis, Mo.) and was dissolved in 5 mM PBS freshly prepared in each instance from solid sodium phosphates. DEX was purchased from American Regent Labs (Shirley, N.Y.). Recombinant mouse IL-6 was purchased from R&D Systems, Inc., Minneapolis, Minn.
IL-6 and TNF-α assays.
Mouse IL-6 concentrations in macrophage culture supernatants, serum, and peritoneal lavage fluid were determined by enzyme-linked immunosorbent assay (ELISA) with the OPTIA IL-6 Set (Pharmingen, San Diego, Calif.).
TNF-α concentrations in macrophage culture supernatants, serum, and peritoneal lavage fluid were determined with the Quantikine M Mouse TNF-α Immunoassay kit (R&D Systems). The minimum concentration of TNF-α stated by the manufacturer to be detectable by this assay is 5.1 pg/ml.
The manufacturer's protocols for both TNF-α and IL-6 assays were followed exactly. All data for TNF-α and IL-6 represent the average of duplicate samples for each specimen. Each experiment was repeated at least twice.
Serum and peritoneal cytokine production.
For the analysis of cytokines in serum and peritoneal lavage fluids, mice were injected i.p. with viable bacteria in 0.2 ml of sterile saline and sacrificed and bled by decapitation with a small-animal guillotine. Serum was separated from clotted blood components and frozen at −70°C until tested. Because of the small volumes, all serum specimens were minimally diluted fivefold prior to testing. Peritoneal lavage was performed immediately after sacrifice by injecting 5.0 ml of PBS into the peritoneal cavity, vigorously agitating the peritoneal cavity, reaspirating the peritoneal lavage fluid, and freezing it at −70°C until testing. Peritoneal cytokine levels are expressed as picograms per peritoneum. This value was determined by measuring the cytokine concentration within the peritoneal lavage fluid and then correcting for the total volume of lavage fluid injected per mouse.
Culture of macrophages.
The murine macrophage-like cell line RAW 264.7 (American Type Culture Collection, Manassas, Va.) was used in all of the in vitro tissue culture studies described here. Macrophages were cultured in RPMI 1640 medium (Life Technologies, Grand Island, N.Y.) supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% heat-inactivated fetal bovine serum (endotoxin content of <0.06 ng/ml; Sigma) at 37°C in a humidified 5% CO2-95% air environment. Before being stimulated, macrophages were seeded in culture plates and cultured overnight.
Sepsis lethality studies.
Mice were injected i.p. with 0.4 ml of LPS (0.125 μg/ml) or a bacterial suspension per mouse with or without 50 mg of d-gal per ml. In some experiments, 100 μg of DEX in 0.2 ml of PBS was also injected i.p. immediately before the bacterial challenge. Lethality was monitored at defined time intervals for up to 48 h. Fifty percent lethal dose (LD50) determinations were done by the method of Reed and Muench (37). When mice were passively immunized against TNF-α, 6.75 × 104 neutralizing units of polyclonal rabbit anti-TNF-α antibody (a kind gift of Roderick McCallum, Texas A&M University, College Station) per mouse was injected via the i.p. route 1 h prior to a bacterial or endotoxin challenge, exactly as previously described; appropriately diluted normal rabbit serum (NRS) was used as a control (21).
Statistics.
TNF-α and IL-6 levels in serum, peritoneal fluid, and culture supernatant fluid are presented as means ± standard errors of the means (SEM), with differences between groups assessed for significance by the Student paired t-test method (10). Differences in mortality were compared with the Fisher exact probability test (40). With each statistical method, a calculated P value of <0.05 was considered significant.
RESULTS
Differential d-gal sensitization of mice to potentially lethal bacterial infections.
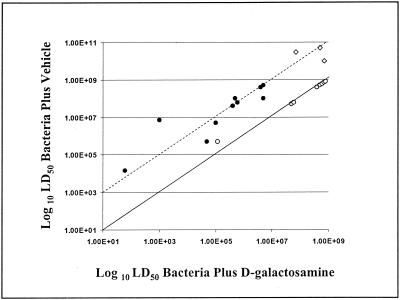
Eleven gram-positive (6 S. aureus strains, 1 S. mitis strain, 1 S. pneumoniae strain, and 3 E. faecalis strains) and 10 gram-negative (3 E. coli strains, 4 K. pneumoniae strains, 1 C. diversus strain, 1 P. aeruginosa strain, and 1 P. mirabilis strain) bacterial strains were evaluated for lethal potency in d-gal-sensitized mice. Figure 1 summarizes the LD50s of all of the bacterial strains tested for untreated mice versus those for mice treated with d-gal. As anticipated, the LD50s of all of the gram-negative bacteria tested were markedly decreased when the animals were treated with d-gal relative to those of the untreated controls (approximately 2 orders of magnitude for most gram-negative isolates). Further, the LD50s of gram-positive bacteria other than E. faecalis were generally unaltered by d-gal treatment relative to those of controls. In striking contrast to these findings, however, the LD50s of all three of the E. faecalis isolates tested were reduced to an extent roughly comparable to those observed with the gram-negative isolates. Thus, our previously published findings suggesting that TNF-α contributes to the lethality induced by E. coli but not to that induced by S. aureus appear to be generally applicable to other gram-positive and gram-negative bacteria, with the exception of E. faecalis (41).
FIG. 1.
Differential d-gal sensitization of mice to lethality induced by clinical and laboratory bacterial isolates. Eleven gram-positive (6 S. aureus strains, 1 S. mitis strain, 1 S. pneumoniae strain, and 3 E. faecalis strains) and 10 gram-negative (3 E. coli strains, 4 K. pneumoniae strains, 1 C. diversus strain, 1 P. aeruginosa strain, and 1 P. mirabilis strain) bacterial strains were evaluated for lethal potency in d-gal-sensitized mice. Animals were injected i.p. with graded doses of each bacterial strain either with or without d-gal, and the resulting mortality was evaluated over 48 h. Each datum point represents experiments performed with at least 48 mice. The solid diagonal line is drawn at a 1:1 ratio between the LD50s of untreated and d-gal-treated mice. The dashed diagonal line is drawn at a 100:1 ratio between the LD50s of untreated and d-gal-treated mice. Consequently, if an isolate is aligned on the solid diagonal line, its LD50 was not changed by d-gal treatment. If, however, an isolate is aligned on the dashed diagonal line, its LD50 was decreased 100-fold by d-gal treatment. Symbols: ⋄, E. faecalis; •, gram-negative bacteria; ○, gram-positive bacteria other than E. faecalis.
DEX protection of mice from potentially lethal bacterial infections.
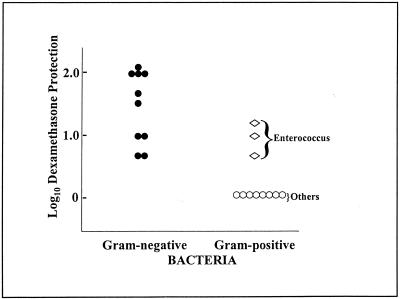
DEX is known to inhibit LPS-induced TNF-α production by mononuclear phagocytes and typically protects mice against the detrimental effects of LPS but does not protect against the lethal effects of TNF-α itself, suggesting that its in vivo effects are mediated primarily through inhibition of TNF-α production (19). This protective effect of DEX can be most clearly documented in d-gal-treated mice, where DEX substantially reduces the extent of d-gal sensitization. The data in Fig. 2 summarize the relative protective effects of DEX against the same gram-negative and gram-positive bacterial isolates described in the legend to Fig. 1. DEX provided protection against a lethal challenge with all of the gram-negative bacteria tested but failed to protect against a lethal challenge with most of the gram-positive bacteria tested (Fig. 2). Once again, however, the three E. faecalis isolates were the exception to these findings; DEX treatment provided survival protection to E. faecalis-infected and d-gal-treated mice. These data provide further support for the conclusion that TNF-α mediates lethality induced by all of the gram-negative isolates tested but does not contribute substantially to lethality induced by the gram-positive bacteria tested, with the exception of the three E. faecalis isolates.
FIG. 2.
DEX protection of mice from lethal infection with clinical and laboratory bacterial isolates. The bacterial strains represented are identical to those described in the legend to Fig. 1. Animals were injected i.p. with graded doses of each bacterial strain with d-gal with or without 100 μg of DEX, and the resulting mortality was evaluated over 48 h. Each datum point represents experiments performed with at least 48 mice. The data show the impact that pretreatment with 100 μg of DEX has on the LD50 of each bacterial isolate tested. Symbols: ⋄, E. faecalis; •, gram-negative bacteria; ○, gram-positive bacteria other than E. faecalis.
Protective effects of anti-TNF-α antibody.
Administration of neutralizing anti-TNF-α antibody to LPS-challenged, d-gal-treated mice is known to provide significant protection against lethality (19). Given the data above suggesting that TNF-α contributes to lethal E. faecalis infection in d-gal-sensitized mice, we investigated whether neutralizing antibody to TNF-α would protect against E. faecalis infection. Control animals were administered NRS, and LPS (0.05 μg per mouse) was injected i.p. as an additional control, both to confirm the protective efficacy of the anti-TNF-α antibody and to document increased sensitization to lethality due to d-gal treatment. The results of two experiments designed to determine the extent to which rabbit polyclonal anti-TNF-α antibody would protect mice from a lethal i.p. inoculation with viable E. faecalis (CP-1) are presented in Table 1. As anticipated, all six LPS-treated mice that were pretreated with NRS plus d-gal died (column 2) whereas all six mice pretreated with NRS plus saline (column 1) survived, indicating that d-gal sensitized mice to LPS-induced lethality (P < 0.005). All six LPS-treated mice that were pretreated with anti-TNF-α plus saline survived (column 3) since they had not been sensitized by d-gal. Further, all six LPS-treated mice pretreated with anti-TNF-α plus d-gal survived (column 4), in contrast to the 100% mortality of the six mice pretreated with NRS plus d-gal (column 2) (P < 0.005); thus, anti-TNF-α protected d-gal-treated mice from LPS-induced lethality. These control experiments confirm that d-gal-treated mice were sensitized to LPS-induced lethality and that anti-TNF-α antibody provides protection to these sensitized mice.
TABLE 1.
Anti-TNF antibody-mediated protection of d-gal-treated mice against lethal injection with either LPS or E. faecalis CP-1a
| Treatment | Mortality
|
|||
|---|---|---|---|---|
| NRS
|
Neutralizing anti-TNF serum
|
|||
| −d-gal | +d-gal | −d-gal | +d-gal | |
| LPS | 0/6 | 6/6b | 0/6 | 0/6b |
| E. faecalis | ||||
| 108 CFU | 0/6 | 0/6 | 0/6 | 0/6 |
| 109 CFU | 0/6 | 6/6b | 0/6 | 0/6b |
| 1010 CFU | 5/6 | 5/6 | 3/6 | 6/6 |
Mice were injected i.p. with 0.4 ml of LPS (0.125 μg/ml) or a bacterial suspension per mouse with or without d-gal at 50 mg/ml. Lethality was monitored for 48 h. Mice were passively immunized against TNF by administering polyclonal rabbit anti-TNF antibody via the i.p. route 1 h prior to a bacterial or endotoxin challenge. Appropriately diluted NRS was used as a control. The numerator represents the number of mice dead at 48 h, and the denominator is the number of mice receiving the specified treatment. The data shown represent a composite of two experiments.
P < 0.005.
Data obtained with E. faecalis CP-1-treated mice given three challenge doses are also presented in Table 1. The key result from this experiment was obtained with an inoculum of 109 CFU/ml. All six of the mice inoculated with 109 CFU of E. faecalis that were pretreated with NRS plus d-gal died (column 2), whereas all six of the mice pretreated with NRS plus saline (column 1) survived, indicating that d-gal sensitized mice to E. faecalis-induced lethality (P < 0.005). All six of the mice inoculated with 109 CFU of E. faecalis that were pretreated with anti-TNF-α plus saline survived (column 3), as anticipated, since they had not been sensitized by treatment with d-gal. All six of the mice inoculated with 109 CFU of E. faecalis that were pretreated with anti-TNF-α plus d-gal survived (column 4), in contrast to the 100% mortality of the six mice pretreated with NRS plus d-gal (column 1) (P < 0.005). Thus, these data confirm that anti-TNF-α also protected d-gal-treated mice from E. faecalis-induced lethality in a manner essentially identical to that observed with LPS-challenged mice. All mice receiving an inoculum of 108 CFU of E. faecalis survived, whereas mice receiving an inoculum of 1010 CFU or greater (data not shown) generally died regardless of whether or not d-gal or anti-TNF-α antibody was administered. Thus, the protective effects of anti-TNF-α antibody are primarily restricted to those mice that are sensitized to the lethal effects of E. faecalis infection by d-gal.
In vivo TNF-α response to E. faecalis infection.
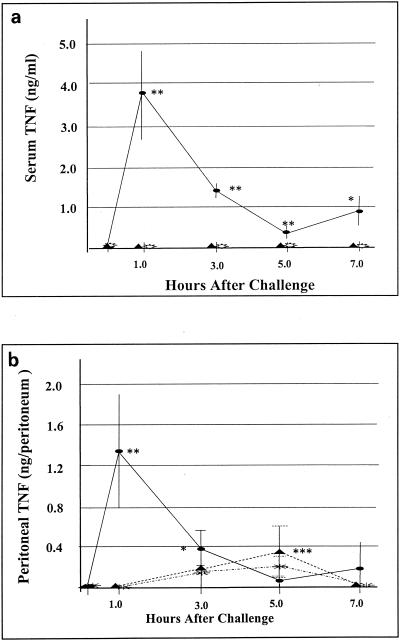
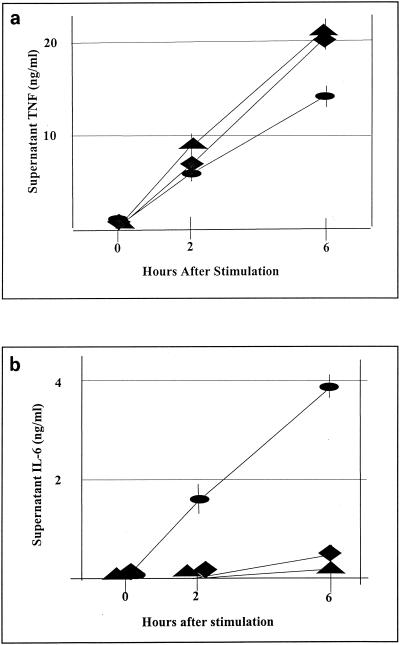
To further investigate the potential contribution of TNF-α to the pathogenesis of enterococcal infections, we infected mice i.p. with two distinct strains of E. faecalis (CP-1 and K9) and measured TNF-α levels in blood and peritoneal fluid at various times after infection; E. coli was used as a control. As shown in Fig. 3a, TNF-α levels in serum peaked 1 h after E. coli infection and decreased thereafter. In contrast, neither strain of E. faecalis induced a detectable systemic TNF-α response. The failure of E. faecalis to induce a systemic TNF-α response was unanticipated in view of studies with d-gal-treated mice, described above, implicating TNF-α in the pathogenesis of lethal E. faecalis infection. We have previously shown that another gram-positive bacterium (i.e., S. aureus) induces a serum TNF-α response that was initially detected (<1 ng/ml) at 3 h and continued to increase until at least 6 h after i.p. infection (42). As shown in Fig. 3b, E. coli also induced a rapid peritoneal TNF-α response, with peak levels 1 h after infection. The peritoneal TNF-α response to E. faecalis was significantly less rapid and pronounced than the response to E. coli, but TNF-α was readily detectable in the peritoneal cavity shortly after E. faecalis infection.
FIG. 3.
TNF-α levels in serum and peritoneal fluid following an i.p. bacterial challenge. Shown are TNF levels in serum and peritoneal fluid following i.p. inoculation with viable E. coli (107 CFU per mouse), E. faecalis K9 (109 CFU per mouse), or E. faecalis CP-1 (109 CFU per mouse). Symbols: ellipses, E. coli; triangles, E. faecalis K9; sunbursts, E. faecalis CP-1. Experiments were performed at least twice with similar results. Each datum point represents the mean ± SEM for five mice. Statistical significance in panel a: **, P < 0.005; *, P < 0.05. Statistical significance in panel b: **, P < 0.005; *, P <0.05 (E. coli versus E. faecalis CP-1); ***, P < 0.05 (E. coli versus E. faecalis K9).
Importantly, 4 (40%) of 10 mice inoculated i.p. with E. faecalis K9 in this experiment died prior to sacrifice, approximately 6 h after inoculation. Peritoneal lavages were performed within an hour of death on three of these four mice, and TNF-α was undetectable in peritoneal lavage fluid from any of the three mice. Additionally, a systemic TNF-α response did not appear to contribute to the observed mortality, as none of the E. faecalis K9-infected mice sacrificed at either 5 or 7 h had detectable circulating TNF-α.
The experiment described above was performed with the expectation of collecting data at 18 h, but all five mice inoculated with E. coli and four of five mice inoculated with E. faecalis K9 died prior to the 18-h specimen collection. In the one E. faecalis K9-infected mouse that survived for 18 h, the peritoneal TNF-α level was determined to be 0.2 ng per peritoneum whereas TNF-α was undetectable in serum and IL-6 was undetectable in the peritoneal fluid and serum. All five mice inoculated with E. faecalis CP-1 survived for 18 h; TNF-α and IL-6 were undetectable in the serum of any of these mice. TNF-α was also undetectable in the peritoneal fluid of all five of these mice, and peritoneal IL-6 levels were 69 ± 33 pg per peritoneum.
These experiments were conducted a total of three times with slight variations in inoculum size and specimen collection times. In the three experiments, 11 (46%) of 24 mice died prior to sacrifice, 5 to 8 h after inoculation with E. faecalis K9, whereas none of 24 mice inoculated with E. faecalis CP-1 died during this time. TNF-α was never detected in the serum of any E. faecalis-infected mouse. Thus, these data support the conclusion that an i.p. challenge with viable E. faecalis can produce mortality in the absence of a detectable systemic TNF-α response.
In vivo IL-6 response to E. faecalis infection.
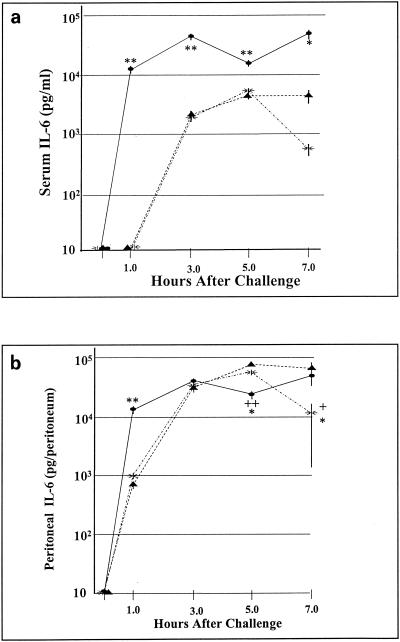
Failure to detect circulating TNF-α in previous experiments prompted further characterization of the host cytokine response. We chose to measure IL-6 levels in plasma and peritoneal fluid following E. faecalis infection because IL-6 levels correlate closely with mortality in sepsis and because IL-6 is considered a sensitive indicator of the host response to infection (20). As shown in Fig. 4a, both E. coli and E. faecalis elicit systemic IL-6 responses. Although E. coli induced significantly higher IL-6 levels in serum (42 ng/ml by 3 h postinoculation) than did E. faecalis (5 ng/ml by 5 h postinoculation), these differences are orders of magnitude less pronounced than the differences observed in the relative TNF-α responses. Within the peritoneal cavity (Fig. 4b), both E. coli and E. faecalis induced potent IL-6 responses, which differed significantly between E. coli and E. faecalis at 1 h postinfection but did not differ significantly by 3 h postinfection. It therefore appears that the cytokine response to an i.p. challenge with E. faecalis, while manifesting marked differences relative to E. coli, as assessed by TNF-α responses, is much less pronounced when IL-6 levels are compared.
FIG. 4.
Serum and peritoneal IL-6 levels following an i.p. bacterial challenge. Shown are serum and peritoneal IL-6 levels following i.p. inoculation with viable E. coli (107 CFU per mouse), E. faecalis K9 (109 CFU per mouse), or E. faecalis CP-1 (109 CFU per mouse). Symbols: ellipses, E. coli; triangles, E. faecalis K9; sunbursts, E. faecalis CP-1. Experiments were performed at least twice with similar results. Each datum point represents the mean ± SEM for five mice. Statistical significance in panel a: **, P < 0.005; *, P < 0.05 (E. coli versus E. faecalis CP-1 and K9). Statistical significance in panel b: **, P < 0.005; ++, P < 0.005 (E. coli versus E. faecalis K9); *, P < 0.05 (E. coli versus E. faecalis CP-1); +, P < 0.05 (E. faecalis CP-1 versus E. faecalis K9).
In vitro cytokine response to E. faecalis.
In view of the atypical host cytokine response to E. faecalis infection in vivo, we investigated the potential for E. faecalis to induce IL-6 and TNF-α production from macrophages in vitro. Preliminary studies indicated that both heat-killed and viable E. faecalis induced a relatively rapid TNF-α response, but only a weak and delayed IL-6 response, from thioglycolate-elicited mouse peritoneal macrophages. To further characterize the in vitro cytokine response to E. faecalis, two of the E. faecalis strains (CP-1 and K9) used in the in vivo studies summarized above were heat killed and added to cultures of the macrophage-like murine cell line RAW 264.7; LPS was used as a control. At various times thereafter, the concentrations of TNF-α and IL-6 in cell culture supernatants were determined by ELISA.
As shown in Fig. 5a, LPS induced the anticipated rapid TNF-α response. Both E. faecalis strains also rapidly induced readily detectable TNF-α, with levels in supernatant cell culture medium exceeding 5 ng/ml by 2 h poststimulation. On the basis of these in vitro results, it appears that the low level of TNF-α in the peritoneal fluid and its undetectability in the serum of mice infected with E. faecalis are not due to an inherent inability of enterococci to induce macrophages to produce TNF-α within the time frame of these in vivo studies.
FIG. 5.
In vitro production of TNF-α and IL-6 by RAW 264.7 cells stimulated with E. faecalis. RAW cells were grown in vitro and stimulated with heat-killed E. faecalis CP-1 or K9 (107 CFU/ml) or LPS (10 ng/ml). Cell culture supernatants were collected at 0, 2, and 6 h after stimulation. IL-6 and TNF concentrations were determined by ELISA. Symbols: ellipses, LPS; triangles, E. faecalis K9; diamonds, E. faecalis CP-1. Experiments were repeated three times with consistent results. Values represent means ± SEM for triplicate tissue culture wells.
As shown in Fig. 5b, the LPS control induced a rapid IL-6 response from macrophages in vitro. With either E. faecalis strain, however, IL-6 levels in supernatant were below the detection limit at 2 h and below 0.5 ng/ml at 6 h after stimulation. Between 6 and 18 h, E. faecalis induced a vigorous IL-6 response, with IL-6 concentrations in supernatant near 40 ng/ml for both CP-1 and K9 (data not shown).
Collectively, the results of these in vitro studies indicate that E. faecalis, in comparison to LPS, can induce relatively rapid and robust TNF-α production but only a delayed IL-6 response in cultures of the RAW 264.7 mouse macrophage-like cell line. These results contrast rather strikingly with in vivo studies reported above that showed that, following an i.p. challenge with E. faecalis, IL-6 was rapidly detectable in both serum and the peritoneal cavity whereas TNF-α was not detected in serum but was detected at low levels in the peritoneal cavity.
IL-6 suppression of in vitro TNF-α production.
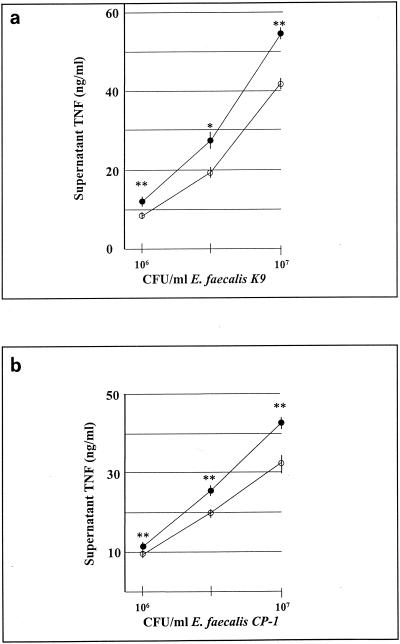
One regulatory function that has been attributed to IL-6 is the ability to suppress an ongoing TNF-α response (20). IL-6 can suppress TNF-α production either directly, by its action on TNF-α-producing cells, or indirectly, by stimulating corticosterone production (3, 4,47). In order to assess the relative ability of IL-6 to directly inhibit TNF-α production by macrophages in response to E. faecalis, RAW cells were pretreated with IL-6 for 18 h prior to E. faecalis stimulation for an additional 18 h. A preliminary dose-response curve was generated with fivefold serial dilutions ranging from 0.08 to 50 ng of IL-6 per ml. These studies showed dose-dependent inhibition of TNF-α production from 0.08 to 2.0 ng/ml with no further inhibition at concentrations greater than 2.0 ng/ml. Subsequent studies were performed with an IL-6 concentration of 10.0 ng of IL-6 per ml to ensure maximum potential suppression. Varying either the period that RAW cells were pretreated with IL-6 from 6 to 18 h or the period that RAW cells were stimulated with E. faecalis from 3 to 18 h did not markedly affect the extent of TNF-α inhibition in subsequent studies.
The data shown in Fig. 6a and b demonstrate a statistically significant, but modest, inhibitory effect of 10 ng of IL-6 per ml on TNF-α production by RAW cells stimulated with various concentrations of E. faecalis. The extent of this suppression would most likely not be sufficient to account for the lack of a detectable systemic TNF-α response to a challenge with E. faecalis in vivo.
FIG. 6.
IL-6-mediated suppression of TNF production by RAW cells. RAW cells were pretreated with medium alone (closed circles) or IL-6 (10 ng/ml) (open circles) for 18 h prior to stimulation with heat-killed E. faecalis K9 (a) or CP-1 (b). Cell culture supernatants were collected after 18 h of stimulation, and TNF concentrations were determined. Experiments were repeated three times with consistent results. Data are means ± SEM. ∗∗, P < 0.005; ∗, P < 0.05.
DISCUSSION
The experiments presented in this report have established that our previously published observations suggesting a differential contribution of TNF-α to lethality due to infection with E. coli or S. aureus in d-gal-treated mice was not unique to those two isolates (41). In this regard, d-gal increased the sensitivity of mice to lethal infection and DEX protected mice against a lethal infection with all 10 of the gram-negative isolates tested, suggesting a central role for TNF-α. In contrast, with all of the gram-positive isolates studied (except those of E. faecalis), d-gal failed to sensitize mice to, and DEX failed to protect them against, lethality, suggesting that TNF-α does not contribute significantly to lethality induced by most gram-positive bacteria in this model. The findings that d-gal sensitized mice to and DEX protected them against the lethal effects of i.p. infection with all three clinical E. faecalis isolates suggest that TNF-α contributes significantly to lethality induced by this organism in the d-gal model. Collectively, these data establish that, in the d-gal model of TNF-α hypersensitization, the results of earlier studies with E. coli and S. aureus would extrapolate to other gram-negative and gram-positive bacteria, with the exception of E. faecalis (41).
Interestingly, however, TNF-α could not be detected in the serum of mice during the first 8 h following i.p. administration of viable E. faecalis. In one experiment, an 18-h time point was included and TNF-α remained undetectable in serum at this time point. TNF-α was detected within the peritonea of mice inoculated i.p. with E. faecalis, but peak peritoneal TNF-α levels were approximately one-sixth to one-third of those observed with E. coli and were delayed by 2 to 4 h.
A particularly interesting finding in these studies was that 11 (46%) of 24 mice infected with E. faecalis K9 died 5 to 8 h after inoculation, whereas none of 24 mice inoculated with E. faecalis CP-1 died in this time frame. The experimental procedures used for both strains were identical, and experiments with both strains were performed simultaneously. Consequently, the differential death rate appears to be attributable to the host's differential response to infection, yet neither strain induced detectable TNF-α levels in serum and peritoneal TNF-α levels were not significantly different between these two strains. In one experiment, three mice that died within 6 h of inoculation with E. faecalis K9 had peritoneal lavages performed within an hour of death; TNF-α was undetectable in peritoneal lavage fluid from any of these mice. As peritoneal TNF-α is generally present 3 h after an i.p. challenge with E. faecalis K9 (Fig. 3b), the absence of detectable TNF-α in peritoneal lavage fluid from these three dead mice arouses curiosity about whether TNF-α might actually protect non-d-gal-treated mice against a potentially lethal enterococcal infection (16, 31, 35). Neutralizing anti-TNF-α antibody also failed to provide significant protection to non d-gal-treated mice infected i.p. with E. faecalis K9 (data not shown). Minimally, these data appear to support the conclusion that TNF-α did not contribute substantially to the death of mice within 5 to 8 h of i.p. inoculation with viable E. faecalis K9. It is extremely important to note that these mice were not pretreated with d-gal.
The absence of a detectable systemic TNF-α response suggests that d-gal sensitization to E. faecalis infection occurs by a totally TNF-α-independent mechanism. Studies with rabbit polyclonal neutralizing anti-TNF-α antibodies, however, would complicate such a conclusion. In this regard, whereas six of six d-gal-treated control mice died following an i.p. challenge with 109 CFU of E. faecalis CP-1, all six d-gal-treated mice pretreated with anti-TNF-α antibody survived an identical bacterial challenge. These results are a composite of two separate experiments and strongly suggest an important role for TNF-α, at least at some level, in lethal E. faecalis infection of d-gal-treated mice. It is noteworthy that the challenge dose of E. faecalis at which anti-TNF-α was most effective was also the dose at which the effects of d-gal are most sensitizing for a lethal E. faecalis infection. Neither d-gal nor anti-TNF-α was shown to have a significant impact on mortality due to inocula of 108, 1010, or 5 × 1010 (data not shown) CFU. Thus, the protective effect of anti-TNF-α antibody was limited to those mice that were sensitized to a lethal E. faecalis infection by d-gal.
Although we have not conclusively excluded the possibility of a systemic TNF-α response at some point beyond 8 h after an E. faecalis infection, the sensitizing effect of d-gal would be expected to have dissipated by this time (11, 15, 43, 49). Additionally, E. faecalis-infected mice tested at 18 h did not have detectable circulating TNF-α and the kinetics of peritoneal TNF-α production indicated a peak at 3 to 5 h, with levels decreasing thereafter. Consequently, late TNF-α production is unlikely to account for the observed d-gal-dependent sensitization of E. faecalis-infected mice. The more likely explanation for the protective effects of anti-TNF-α antibody in d-gal-sensitized, E. faecalis-infected mice is that localized TNF-α production, perhaps within the peritoneal cavity, contributes substantially to mortality. Recent publications support the concept of a potentially lethal compartmentalized cytokine response following intraabdominal infection (30, 39). For E. faecalis K9, mean peritoneal TNF-α levels approximated 200 pg per peritoneum at 3 h postchallenge with 109 bacteria. This level of TNF-α was estimated by injecting 5 ml of PBS into the peritoneum, reaspirating the fluid, determining the TNF-α concentration in the fluid, and multiplying by the volume of lavage fluid. If one considers that there might be only 0.2 ml of fluid in the peritoneum prior to lavaging, however, the concentration of TNF-α within the peritoneal cavity could approach 1.0 ng/ml, which might be sufficient to kill a d-gal-sensitized mouse. Although peritoneal TNF-α levels were not determined in mice challenged with E. faecalis inocula greater than 109 CFU, TNF-α remained undetectable in the serum of mice challenged i.p. with 1010 CFU of E. faecalis CP-1 or K9 (data not shown).
Experiments to explore the kinetics of TNF-α production were carried out with mice that had not been treated with d-gal. We considered the possibility that d-gal treatment itself might enhance TNF-α production in response to E. faecalis, although it is generally accepted that this does not occur with gram-negative bacteria. The validation of such a hypothesis would potentially serve to explain many of the unusual findings presented in this report. Experiments designed to explore this possibility, however, established that d-gal treatment had neither a significant nor a discernible impact on TNF-α production in mice inoculated i.p. with viable E. faecalis (data not shown).
Despite the failure of E. faecalis to induce a systemic TNF-α response, we confirmed that E. faecalis infection elicits a systemic cytokine response by measuring IL-6 levels in serum. Serum IL-6 levels peaked at approximately 5 ng/ml 5 h after i.p. infection with E. faecalis; these levels were approximately one-eighth of the peak IL-6 levels observed 3 h after i.p. infection with E. coli. Within the peritoneal cavity, E. faecalis also induced rapid production of IL-6. Interestingly, by 3 h after a challenge, peritoneal IL-6 production did not differ significantly between E. faecalis and E. coli.
TNF-α is generally considered to be a major stimulus of the in vivo IL-6 response to either LPS or E. coli (46). Others have demonstrated this by showing that pretreatment with anti-TNF-α antibodies essentially abrogates the IL-6 response to LPS or E. coli (17, 45). Our findings that E. faecalis induces a systemic IL-6 response without a systemic TNF-α response and that IL-6 is detected prior to TNF-α in peritoneal fluid suggest that in vivo, E. faecalis induces IL-6 by a TNF-α-independent mechanism. This finding may be significant with respect to modulation of the host cytokine response.
The relatively modest in vivo TNF-α response elicited by E. faecalis was somewhat unanticipated, in view of published reports that lipoteichoic acid purified from E. faecalis has the capacity to induce TNF-α production from macrophages in vitro (5, 24). Studies done to assess the capacity of E. faecalis CP-1 and K9 to induce TNF-α production from thioglycolate-elicited mouse peritoneal macrophages indicated that both viable and heat-killed organisms are capable of promoting TNF-α secretion. Further studies conducted with heat-killed E. faecalis and the murine macrophage-like cell line RAW 264.7 indicated that E. faecalis repeatedly and consistently induced significant TNF-α production in vitro within 2 h of a challenge but that IL-6 production was typically delayed for several hours.
In view of the failure of E. faecalis to induce an early systemic TNF-α response in vivo, the capacity of E. faecalis to induce an early TNF-α response in vitro is intriguing. We considered the possibility that E. faecalis might promote suppression of a TNF-α response in vivo. There are many possible mechanisms by which this could occur, and it was beyond the scope of this study to exhaustively explore these possibilities. In this regard, one regulatory function attributed to IL-6 is the ability to suppress an ongoing TNF-α response (3, 20). In the typical response to a bacterial infection or LPS, TNF-α production normally precedes IL-6 production (32, 44). The early release of TNF-α stimulates subsequent IL-6 production, which then functions as part of a negative-feedback regulatory pathway that turns off TNF-α production (12, 17, 25, 45). IL-6 can suppress TNF-α production either directly, by its action on TNF-α-producing cells, or indirectly, by stimulating corticosterone production (3, 4,47). Our data indicate that the in vivo response of mice to E. faecalis infection differs from the response to gram-negative bacteria (e.g., E. coli) and LPS, in that circulating IL-6 levels are elevated soon after a bacterial challenge, despite undetectable TNF-α in plasma. Under these conditions, it is possible that early and sustained IL-6 production might suppress TNF-α production before significant levels of TNF-α can be generated. Such a mechanism might be anticipated to abort a potential systemic TNF-α response to E. faecalis infection. Consequently, in vitro studies were conducted and established that pretreatment of RAW cells with IL-6 did, in fact, suppress TNF-α production in response to E. faecalis. Despite the statistical significance of these findings, the extent to which TNF-α production was suppressed represented only a relatively modest reduction in the total TNF-α produced. Thus, although these data suggest that IL-6 might contribute somewhat to the weak in vivo TNF-α response to E. faecalis infection, they do not support the conclusion that IL-6 is fully responsible for this effect. It is possible, however, that the ability of IL-6 to inhibit TNF-α production by macrophages stimulated with E. faecalis is only partially attributable to its direct interaction with macrophages and that IL-6-mediated TNF-α suppression might be enhanced in vivo (4, 47).
In summary, the data presented here support the following key conclusions. First, d-gal sensitization to E. faecalis-induced mortality appears to occur by a TNF-α-dependent mechanism in the absence of a systemic TNF-α response, thereby suggesting that localized TNF-α production, perhaps within the peritoneal cavity, contributes substantially to fatal infection in this model. Second, in mice that had not been treated with d-gal, the pathogenesis of an acute fatal infection following i.p. inoculation with E. faecalis appears to occur via a TNF-α-independent mechanism. Third, the relatively modest TNF-α response to E. faecalis infection in vivo, in conjunction with the capacity of E. faecalis to elicit a robust TNF-α response in vitro, suggests that there may be active suppression of TNF-α production in response to E. faecalis infection in vivo. Fourth, it appears likely that E. faecalis induces early systemic and peritoneal IL-6 production through a largely TNF-α-independent mechanism. Finally, pretreatment of macrophages with IL-6 results in significant suppression of TNF-α production following stimulation with E. faecalis, but this suppression appears to be insufficient to account for the feeble in vivo TNF-α response to E. faecalis infection. Collectively, the data generated in this study further emphasize the heterogeneous nature of the host inflammatory cytokine response to infectious stimuli. Acute lethal E. faecalis infection appears to induce an unusual cytokine response that, when further characterized and understood, will provide significant additional insights into the pathogenesis of sepsis.
Acknowledgments
This work was supported by Public Health Service grant R15-AI46493 from the National Institute of Allergy and Infectious Diseases and by a grant from the Sarah Morrison Bequest.
We acknowledge Roderick McCallum and Nilofer Qureshi with gratitude for the kind gifts of anti-TNF-α antibody and LPS, respectively. We also acknowledge the expert technical assistance of Qiao Xue, Rashel Mostafavi, and Eleanor Zuvanich.
Editor: J. T. Barbieri
REFERENCES
- 1.Abraham, E. 1999. Why immunomodulatory therapies have not worked in sepsis. Intensive Care Med. 25:556-566. [DOI] [PubMed] [Google Scholar]
- 2.Abraham, E., A. Anzueto, G. Gutierrez, S. Tessler, G. San Pedro, R. Wunderlink, A. Dal Nogare, S. Nasraway, S. Berman, R. Coonery, H. Levy, R. Baughman, M. Rumbak, R. B. Light, L. Poole, R. Allred, J. Constant, J. Pennington, and S. Porter. 1998. Double blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. Lancet 351:929-933. [PubMed] [Google Scholar]
- 3.Aderka, D., J. Le, and J. Vilcek. 1989. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J. Immunol. 143:3517-3523. [PubMed] [Google Scholar]
- 4.Bethin, K. E., S. K. Vogt, and L. J. Muglia. 2000. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc. Natl. Acad. Sci. USA 97:9317-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakdi, S., T. Klonisch, P. Nuber, and W. Fischer. 1991. Stimulation of monokine production by lipoteichoic acids. Infect. Immun. 59:4614-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bone, R. C. 1994. Gram-positive organisms and sepsis. Arch. Intern. Med. 154:26-34. [PubMed] [Google Scholar]
- 7.Bone, R. C. 1997. Important new findings in sepsis. JAMA 278:249.. [PubMed] [Google Scholar]
- 8.Bone, R. C., C. J. Grodzin, and R. A. Balk. 1997. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest 112:235-243. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, J., J. Carlet, et al. 1996. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-α in patients with sepsis. Crit. Care Med. 24:1431-1440. [DOI] [PubMed] [Google Scholar]
- 10.Croxton, F. E. 1953. Elementary statistics with applications in medicine, p. 235-239 and 326-327. Prentice-Hall, Inc., Englewood Cliffs, N.J.
- 11.Decker, K., and D. Keppler. 1974. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev. Physiol. Biochem. Pharmacol. 71:77-106. [DOI] [PubMed] [Google Scholar]
- 12.Di Santo, E., T. Alonzi, V. Poli, E. Fattori, C. Toniatti, M. Sironi, P. Ricciardi-Castagnoli, and P. Ghezzi. 1997. Differential effects of IL-6 on systemic and central production of TNF: a study with IL-6-deficient mice. Cytokine 9:300-306. [DOI] [PubMed] [Google Scholar]
- 13.Edmond, M. B., J. F. Ober, J. D. Dawson, D. L. Weinbaum, and R. P. Wenzel. 1996. Vancomycin-resistant enterococcal bacteremia: natural history and attributable mortality. Clin. Infect. Dis. 23:1234-1239. [DOI] [PubMed] [Google Scholar]
- 14.Eigler, A., B. Sinha, G. Hartmann, and S. Endres. 1997. Taming TNF: strategies to restrain this proinflammatory cytokine. Immunol. Today 18:487-492. [DOI] [PubMed] [Google Scholar]
- 15.Endo, Y., M. Shibazaki, K. Yamaguchi, K. Kai, S. Sugawara, H. Takada, H. Kikuchi, and K. Kumagai. 1999. Enhancement by galactosamine of lipopolysaccharide (LPS)-induced tumour necrosis factor production and lethality: its suppression by LPS pretreatment. Br. J. Pharmacol. 128:5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher, C. J., Jr., J. M. Agosti, S. M. Opal, S. F. Lowry, R. A. Balk, J. C. Sadoff, E. Abraham, R. M. H. Schein, and E. Benjamin. 1996. Treatment of septic shock with tumor necrosis factor receptor:Fc fusion protein. N. Engl. J. Med. 334:1697-1702. [DOI] [PubMed] [Google Scholar]
- 17.Fong, Y., K. J. Tracey, L. L. Moldawer, D. G. Hesse, K. B. Manogue, J. S. Kenney, A. T. Lee, G. C. Kuo, A. C. Allison, S. F. Lowry, and A. Cerami. 1989. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1β and interleukin 6 appearance during lethal bacteremia. J. Exp. Med. 170:1627-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freudenberg, M. A., and C. Galanos. 1991. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in d-galactosamine-treated mice. Infect. Immun. 59:2110-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galanos, C., and M. A. Freudenberg. 1990. Tumor necrosis factor mediates endotoxin shock: the protective effects of antibodies and cortisone, p. 187-193. In B. Bonavida and G. Granger (ed.), Tumor necrosis factor: structure, mechanism of action, role in disease and therapy. Karger, Basel, Switzerland.
- 20.Hack, C. E., L. A. Aarden, and L. G. Thijs. 1997. Role of cytokines in sepsis. Adv. Immunol. 66:101-195. [DOI] [PubMed] [Google Scholar]
- 21.Hill, M. R., and R. E. McCallum. 1992. Identification of tumor necrosis factor as a transcriptional regulator of the phosphoenolpyruvate caboxykinase gene following endotoxin treatment of mice. Infect. Immun. 60:4040-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jett, B. D., M. K. Huycke, and M. S. Gilmore. 1994. Virulence of enterococci. Clin. Microbiol. Rev. 7:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, A. P. 1994. The pathogenicity of enterococci. J. Antimicrob. Chemother. 33:1083-1089. [DOI] [PubMed] [Google Scholar]
- 24.Keller, R., W. Fischer, R. Keist, and S. Bassetti. 1992. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect. Immun. 60:3664-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozak, W., M. J. Kluger, D. Sosynski, C. A. Conn, K. Rudolph, L. R. Leon, and H. Zheng. 1998. IL-6 and Il-1β in fever. Studies using cytokine-deficient (knockout) mice. Ann. N. Y. Acad. Sci. 856:33-47. [DOI] [PubMed] [Google Scholar]
- 26.Landry, S. L., D. L. Kaiser, and R. P. Wenzel. 1989. Hospital stay and mortality attributed to nosocomial enterococcal bacteremia: a controlled study. Am. J. Infect. Control 17:323-329. [DOI] [PubMed] [Google Scholar]
- 27.Leclercq, R. 1997. Enterococci acquire new kinds of resistance. Clin. Infect. Dis. 24(Suppl. 1):S80-S84. [DOI] [PubMed] [Google Scholar]
- 28.Le Roy, D., P. Morand, S. Lengacher, M. Celio, G. E. Grau, M. P. Glauser, and D. Heumann. 1996. Streptococcus mitis cell walls and lipopolysaccharide induce lethality in d-galactosamine-sensitized mice by a tumor necrosis factor-dependent pathway. Infect. Immun. 64:1846-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynn, W. A. 1998. Anti-endotoxin therapeutic options for the treatment of sepsis. J. Antimicrob. Chemother. 41(Suppl. A):71-80. [DOI] [PubMed] [Google Scholar]
- 30.Martineau, L., and P. N. Shek. 2000. Peritoneal cytokine concentrations and survival outcome in an experimental bacterial infusion model of peritonitis. Crit. Care Med. 28:788-794. [DOI] [PubMed] [Google Scholar]
- 31.Nakane, A., T. Minagawa, and K. Kato. 1988. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect. Immun. 56:2563-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakane, A., M. Okamoto, M. Asano, M. Khoanawa, and T. Minagawa. 1995. Endogenous gamma interferon, tumor necrosis factor, and interleukin-6 in Staphylococcus aureus infection in mice. Infect. Immun. 63:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowak, M., G. C. Gaines, J. Rosenberg, R. Minter, F. R. Bahjat, J. Rectenwald, S. L. MacKay, C. K. Edwards III, and L. L. Moldawer. 2000. LPS-induced liver injury in d-galactosamine-sensitized mice requires secreted TNF-alpha and the TNF-p55 receptor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278:R1202-R1209. [DOI] [PubMed] [Google Scholar]
- 34.Opal, S. M., and J. Cohen. 1999. Clinical Gram-positive sepsis: does it fundamentally differ from Gram-negative bacterial sepsis? Crit. Care Med. 27:1608-1616. [DOI] [PubMed] [Google Scholar]
- 35.Pfeffer, K., T. Matsuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73:457-467. [DOI] [PubMed] [Google Scholar]
- 36.Qureshi, N., K. Takayama, P. Mascagni, J. Honovich, R. Wong, and R. J. Cotter. 1988. Complete structural determination of lipopolysaccharide obtained from deep rough mutant of Escherichia coli. Purification by high performance liquid chromatography and direct analysis by plasma desorption mass spectrometry. J. Biol. Chem. 263:11971-11976. [PubMed] [Google Scholar]
- 37.Reed, L. J., and H. A. Muench. 1938. A simple method of estimating 50 percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 38.Reinhart, K., C. Wiegand-Lohnert, F. Grimminger, M. Kaul, S. Withington, D. Treacher, J. Eckart, S. Willatts, C. Bouza, D. Krausch, F. Stockenhuber, J. Eiselstein, L. Daum, and J. Kempeni. 1996. Assessment of the safety and efficacy of the monoclonal anti-tumor necrosis factor antibody-fragment, MAK 195F, in patients with sepsis and septic shock: a multicenter, randomized, placebo-controlled, dose-ranging study. Crit. Care Med. 24:733-742. [DOI] [PubMed] [Google Scholar]
- 39.Schien, M. D. H. Wittmann, R. Holzheimer, and R. E. Condon. 1996. Hypothesis: compartmentalization of cytokines in intraabdominal infection. Surgery 119:694-699. [DOI] [PubMed] [Google Scholar]
- 40.Siegel, S. 1956. Nonparametric statistics, p. 96-100. McGraw-Hill Book, Co. New York, N.Y.
- 41.Silverstein, R., M. Norimatsu, and D. C. Morrison. 1997. Fundamental differences during gram-positive versus gram-negative sepsis become apparent during bacterial challenge of d-galactosamine-treated mice. J. Endotoxin Res. 4:173-181. [Google Scholar]
- 42.Silverstein, R., J. G. Wood, Q. Xue, M. Norimatsu, D. L. Horn, and D. C. Morrison. 2000. Differential host inflammatory responses to viable versus antibiotic-killed bacteria in experimental microbial sepsis. Infect. Immun. 68:2301-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sriskandan, S., and J. Cohen. 1999. Gram-positive sepsis. Mechanisms and differences from Gram-negative sepsis. Infect. Dis. Clin. N. Am. 13:397-412. [DOI] [PubMed] [Google Scholar]
- 44.van der Poll, T., and S. J. H. van Deventer. 1999. Cytokines and anticytokines in the pathogenesis of sepsis. Infect. Dis. Clin. N. Am. 13:413-426. [DOI] [PubMed] [Google Scholar]
- 45.van der Poll, T., M. Levi, S. J. H. van Deventer, H. ten Cate, B. L. Haagmans, B. J. Biemond, H. R. Buller, C. E. Hack, and J. W. ten Cate. 1994. Differential effects of anti-tumor necrosis factor monoclonal antibodies on systemic inflammatory responses in experimental endotoxemia in chimpanzees. Blood 83:446-451. [PubMed] [Google Scholar]
- 46.van der Poll, T., and S. J. H. van Deventer. 1998. The role of interleukin 6 in endotoxin-induced inflammatory responses. Prog. Clin. Biol. Res. 397:365-377. [PubMed] [Google Scholar]
- 47.Van Enckevort, F. H. J., D. G. J. Sweep, P. N. Span, M. G. Netea, A. R. M. M. Hermus, and B. J. Kullberg. 2001. Reduced adrenal response and increased mortality after systemic Klebsiella pneumoniae infection in interleukin-6-deficient mice. Eur. Cytokine Netw. 12:581-586. [PubMed] [Google Scholar]
- 48.Wenzel, R. P. 1992. Anti-endotoxin monoclonal antibodies: a second look. N. Engl. J. Med. 326:1151-1153. [DOI] [PubMed] [Google Scholar]
- 49.Wielockx, B., T. Hochepied, J. Staelens, W. Van Molle, P. Brouckaert, and C. Libert. 2001. Detection, characterisation and purification of a murine liver factor capable of desensitising towards the lethal activity of tumour necrosis factor. Cytokine 15:59-65. [DOI] [PubMed] [Google Scholar]