Abstract
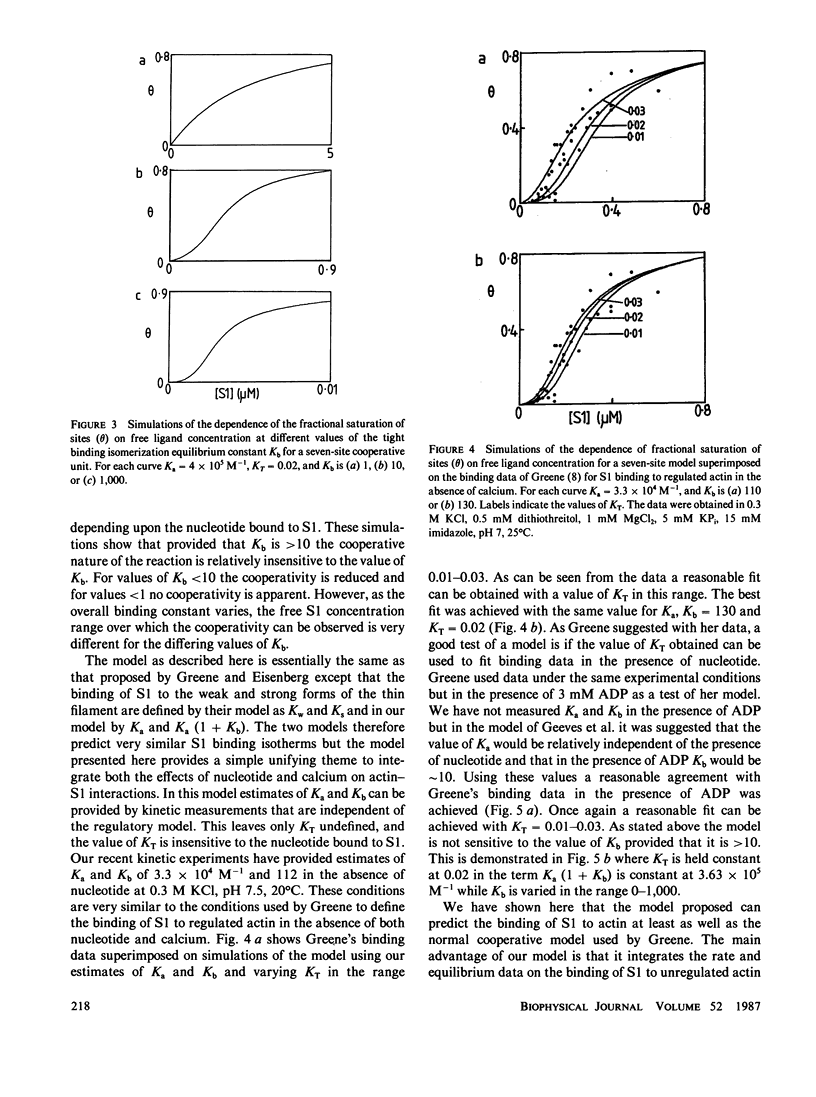
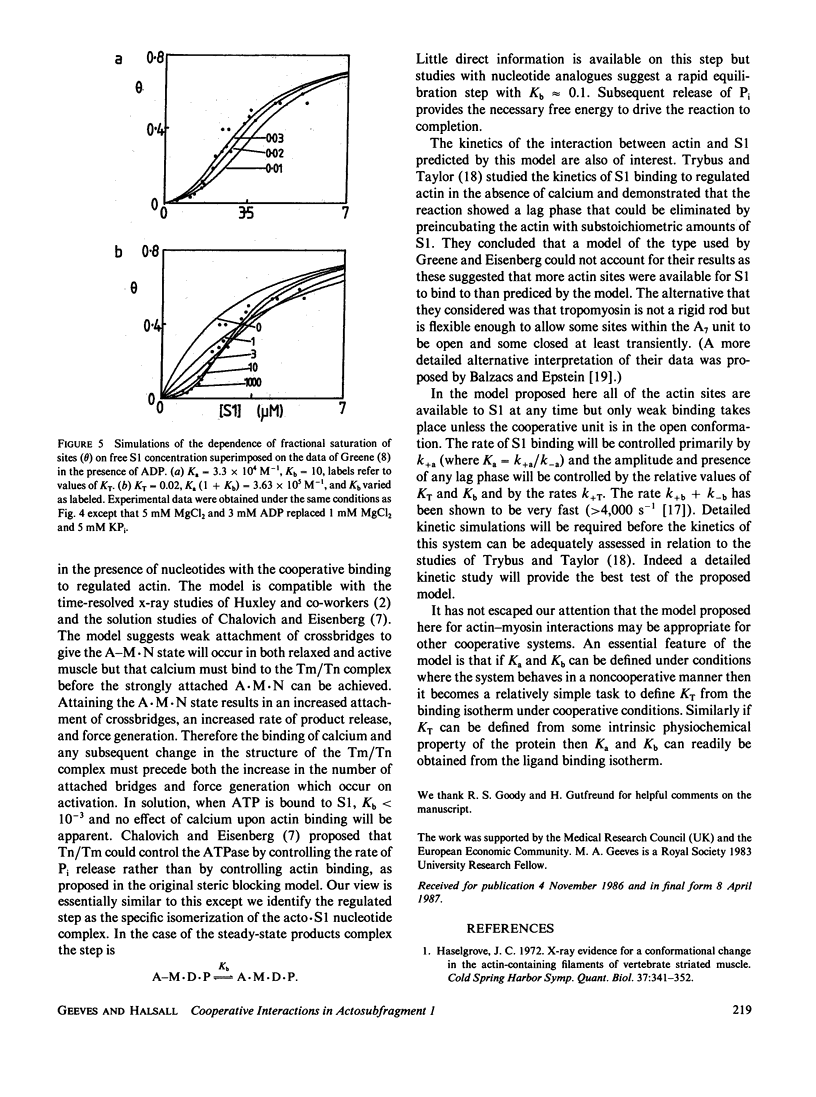
The binding of actin to myosin subfragment 1 (S1) has been shown to occur as a two-step reaction. In the first step actin is weakly bound and then the complex isomerizes to the "rigor type" acto-S1 complex (Coates, J. H., A. H. Criddle, and M. A. Geeves, 1985 Biochem. J., 232:351-356). We propose here a model in which troponin/tropomyosin (Tn/Tm) controls the actin-S1 interaction by inhibiting the isomerization step. In this model the (actin)7 Tn/Tm unit is assumed to exist in two states: open and closed. S1 can bind to either of the two states but only the open form allows the isomerization reaction to take place. We demonstrate that this model can account for the cooperative binding of S1 and S1 nucleotide complexes to actin. The model provides a way of integrating both the effects of calcium and nucleotide on actin-S1 interactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Balazs A. C., Epstein I. R. Kinetic model for the interaction of myosin subfragment 1 with regulated actin. Biophys J. 1983 Nov;44(2):145–151. doi: 10.1016/S0006-3495(83)84286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Coates J. H., Criddle A. H., Geeves M. A. Pressure-relaxation studies of pyrene-labelled actin and myosin subfragment 1 from rabbit skeletal muscle. Evidence for two states of acto-subfragment 1. Biochem J. 1985 Dec 1;232(2):351–356. doi: 10.1042/bj2320351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves M. A., Goody R. S., Gutfreund H. Kinetics of acto-S1 interaction as a guide to a model for the crossbridge cycle. J Muscle Res Cell Motil. 1984 Aug;5(4):351–361. doi: 10.1007/BF00818255. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Halsall D. J. The dynamics of the interaction between myosin subfragment 1 and pyrene-labelled thin filaments, from rabbit skeletal muscle. Proc R Soc Lond B Biol Sci. 1986 Oct 22;229(1254):85–95. doi: 10.1098/rspb.1986.0076. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Jeffries T. E., Millar N. C. ATP-induced dissociation of rabbit skeletal actomyosin subfragment 1. Characterization of an isomerization of the ternary acto-S1-ATP complex. Biochemistry. 1986 Dec 30;25(26):8454–8458. doi: 10.1021/bi00374a020. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 May;77(5):2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. The effect of nucleotide on the binding of myosin subfragment 1 to regulated actin. J Biol Chem. 1982 Dec 10;257(23):13993–13999. [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress M., Huxley H. E., Faruqi A. R., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Squire J. M. Structural role of tropomyosin in muscle regulation: analysis of the x-ray diffraction patterns from relaxed and contracting muscles. J Mol Biol. 1973 Mar 25;75(1):33–55. doi: 10.1016/0022-2836(73)90527-5. [DOI] [PubMed] [Google Scholar]
- Rosenfeld S. S., Taylor E. W. Kinetic studies of calcium binding to regulatory complexes from skeletal muscle. J Biol Chem. 1985 Jan 10;260(1):252–261. [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Kinetic studies of the cooperative binding of subfragment 1 to regulated actin. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7209–7213. doi: 10.1073/pnas.77.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]


