Abstract
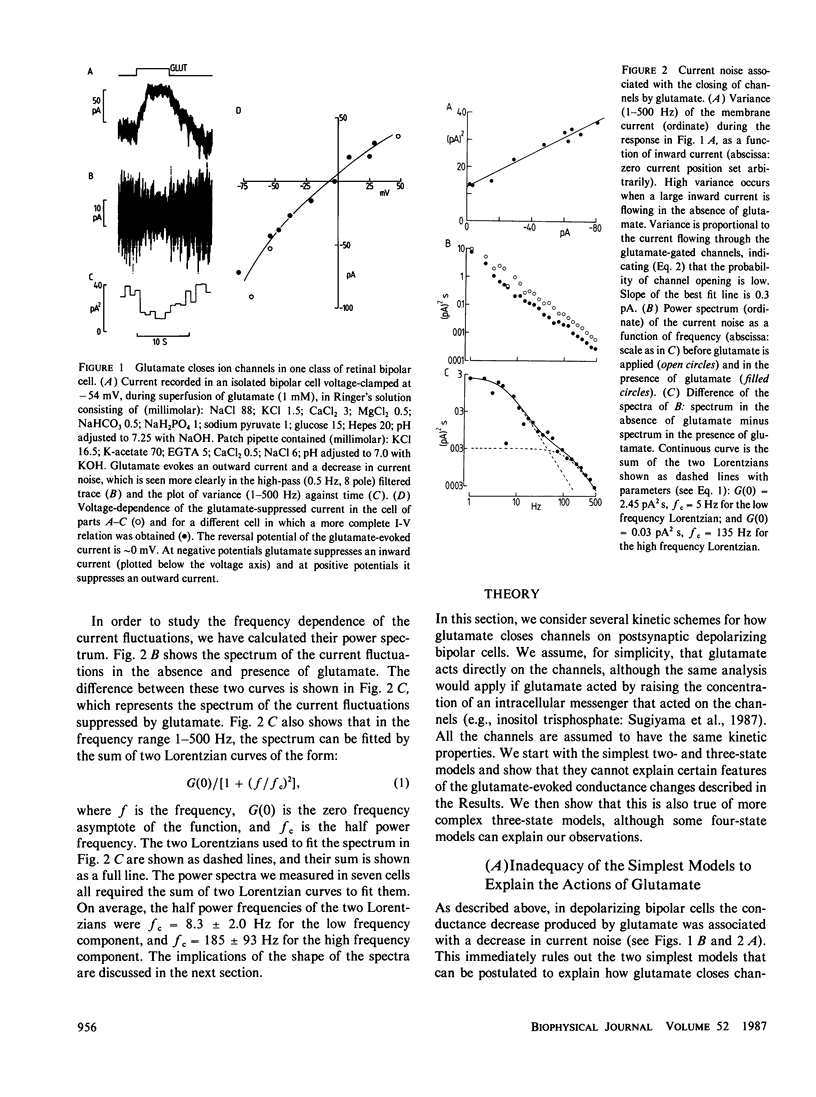
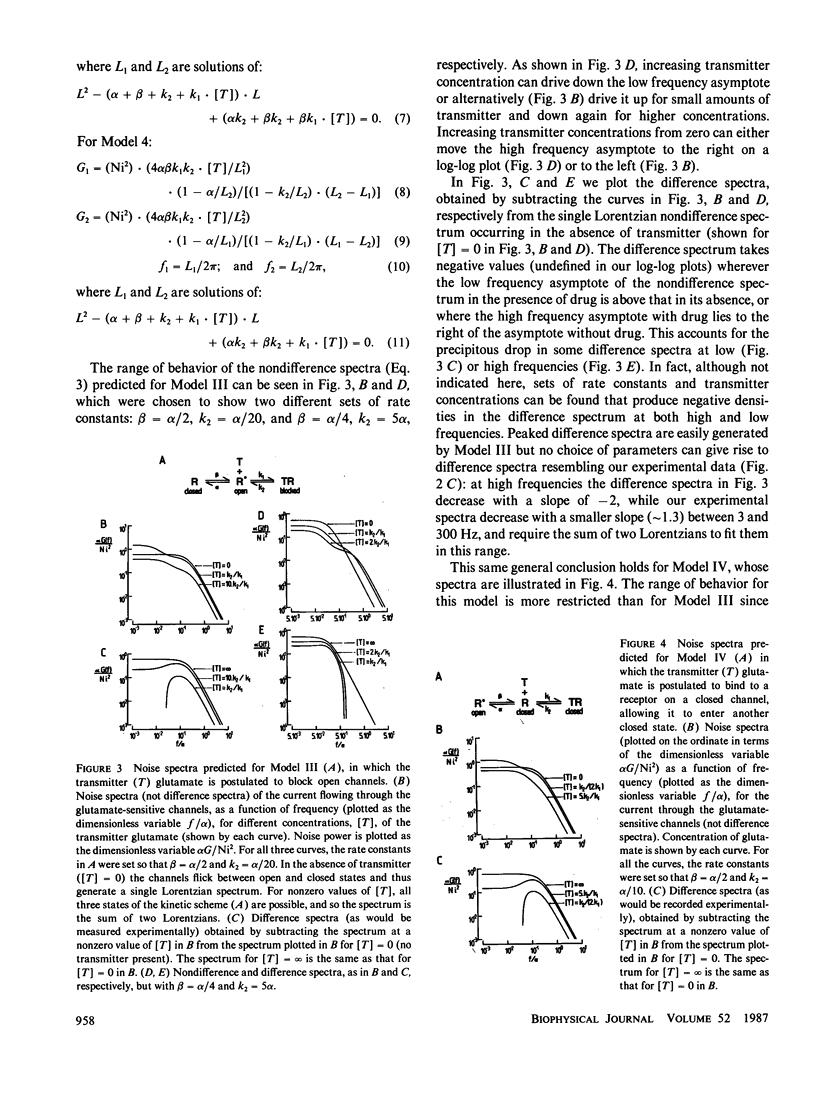
For ion channels that are opened by neurotransmitters, analysis of current noise has given valuable information on the kinetics of synaptic channel gating. In depolarizing bipolar cells of the vertebrate retina, we have recently characterized a synaptic current for which the neurotransmitter glutamate closes channels, and for which the channel open probability is low even in the absence of glutamate. We present here predictions for the current noise spectrum expected for various models of glutamate's action on the ion channels. Comparison of these theoretical predictions with experimental data allows us to rule out several simple kinetic schemes for the action of glutamate, and to conclude that the channels closed by glutamate must be able to exist in at least four different states.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Falk G. Responses of rod bipolar cells in the dark-adapted retina of the dogfish, Scyliorhinus canicula. J Physiol. 1980 Mar;300:115–150. doi: 10.1113/jphysiol.1980.sp013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Mobbs P., Tessier-Lavigne M., Wilson M. Neurotransmitter-induced currents in retinal bipolar cells of the axolotl, Ambystoma mexicanum. J Physiol. 1987 Jun;387:125–161. doi: 10.1113/jphysiol.1987.sp016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological studies of single retinal cells and their morphological identification. Vision Res. 1971;Suppl 3:17–26. doi: 10.1016/0042-6989(71)90027-7. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Effects of L-glutamate on the anomalous rectifier potassium current in horizontal cells of Carassius auratus retina. J Physiol. 1985 Jan;358:169–182. doi: 10.1113/jphysiol.1985.sp015546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S., Copenhagen D. R. Multiple classes of glutamate receptor on depolarizing bipolar cells in retina. Nature. 1987 Jan 1;325(6099):56–58. doi: 10.1038/325056a0. [DOI] [PubMed] [Google Scholar]
- Saito T., Kondo H., Toyoda J. Rod and cone signals in the on-center bipolar cell: their different ionic mechanisms. Vision Res. 1978;18(5):591–595. doi: 10.1016/0042-6989(78)90208-0. [DOI] [PubMed] [Google Scholar]
- Shiells R. A., Falk G., Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981 Dec 10;294(5841):592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. Characterization of an extended glutamate receptor of the on bipolar neuron in the vertebrate retina. J Neurosci. 1985 Jan;5(1):224–233. doi: 10.1523/JNEUROSCI.05-01-00224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985 Aug 22;316(6030):736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Toyoda J. Membrane resistance changes underlying the bipolar cell response in the carp retina. Vision Res. 1973 Feb;13(2):283–294. doi: 10.1016/0042-6989(73)90107-7. [DOI] [PubMed] [Google Scholar]


