Abstract
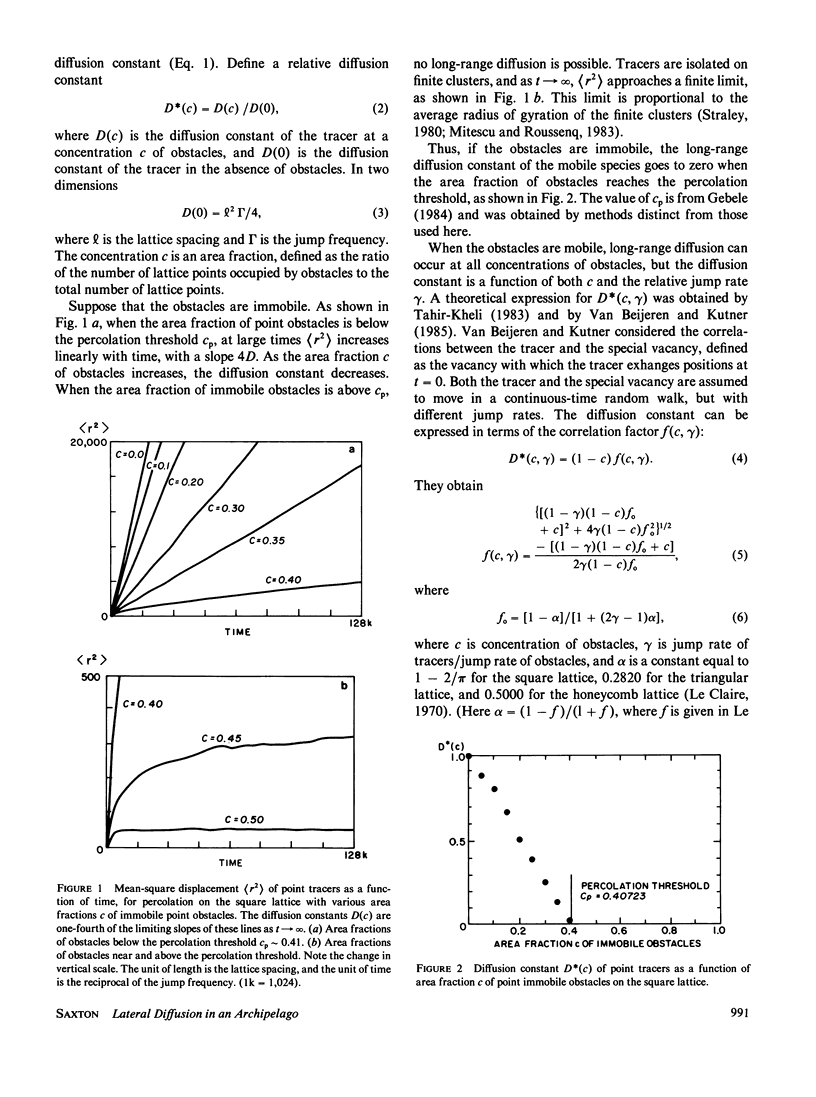
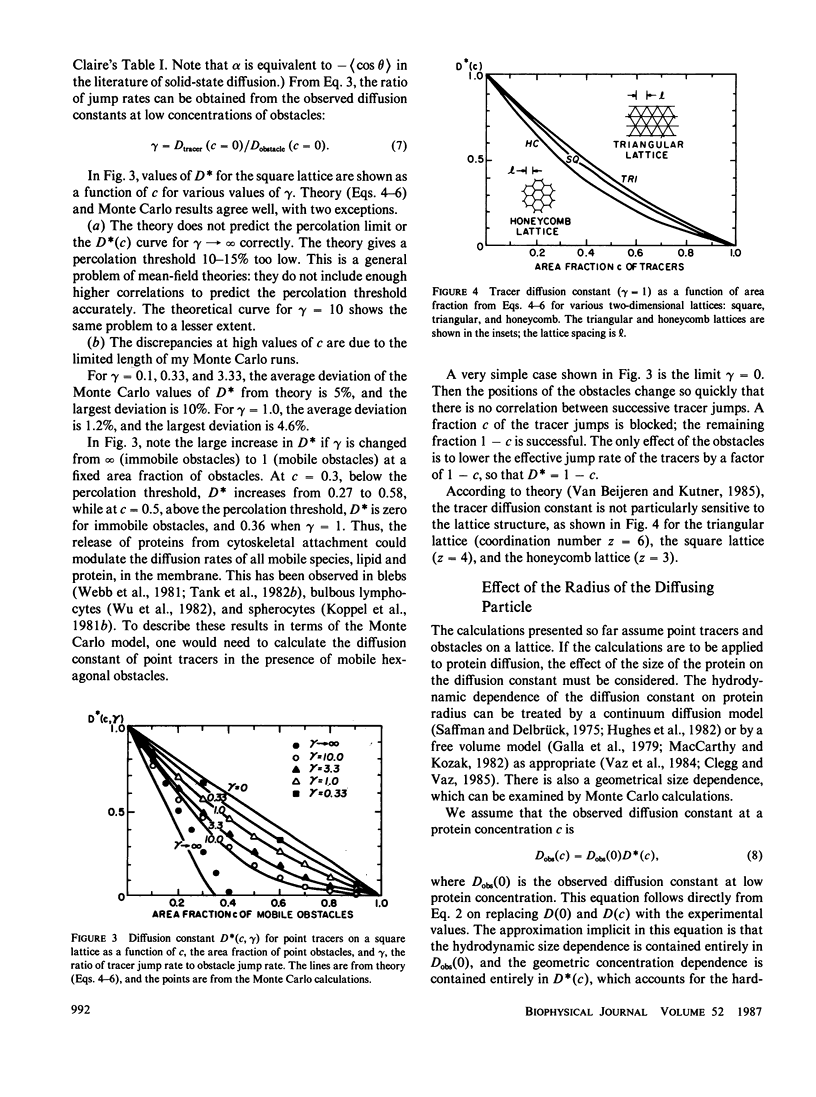
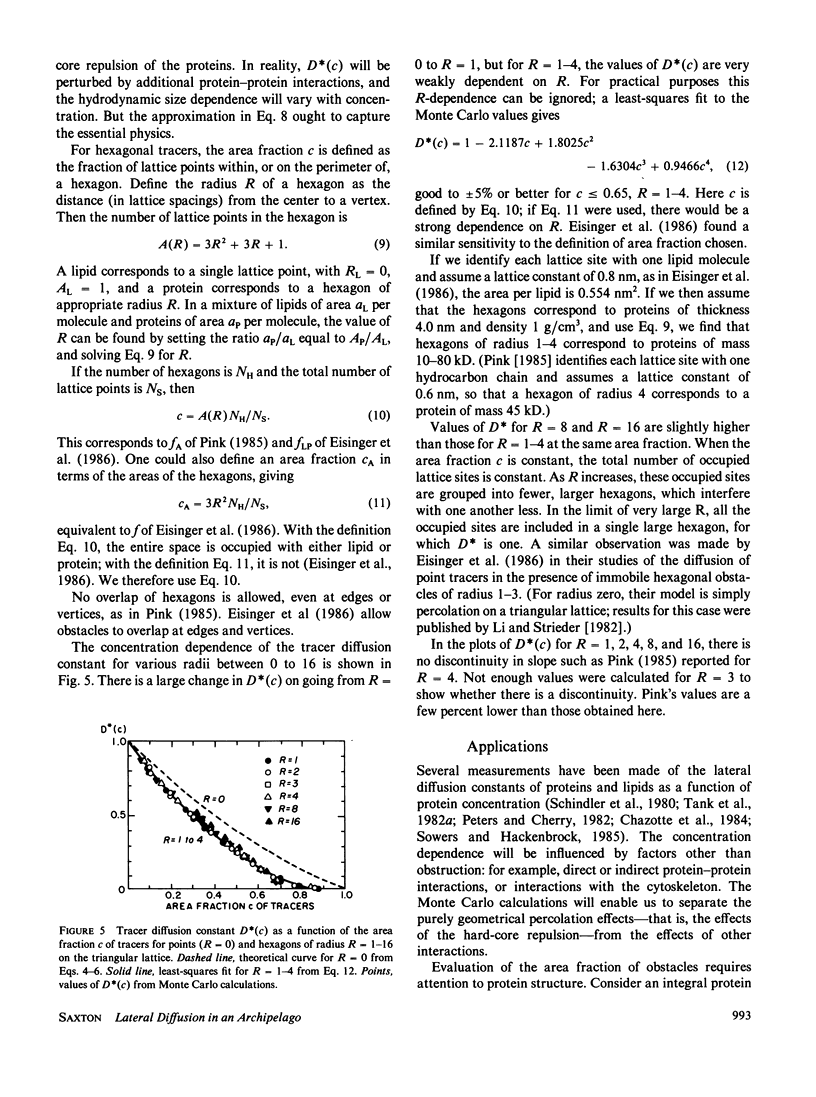
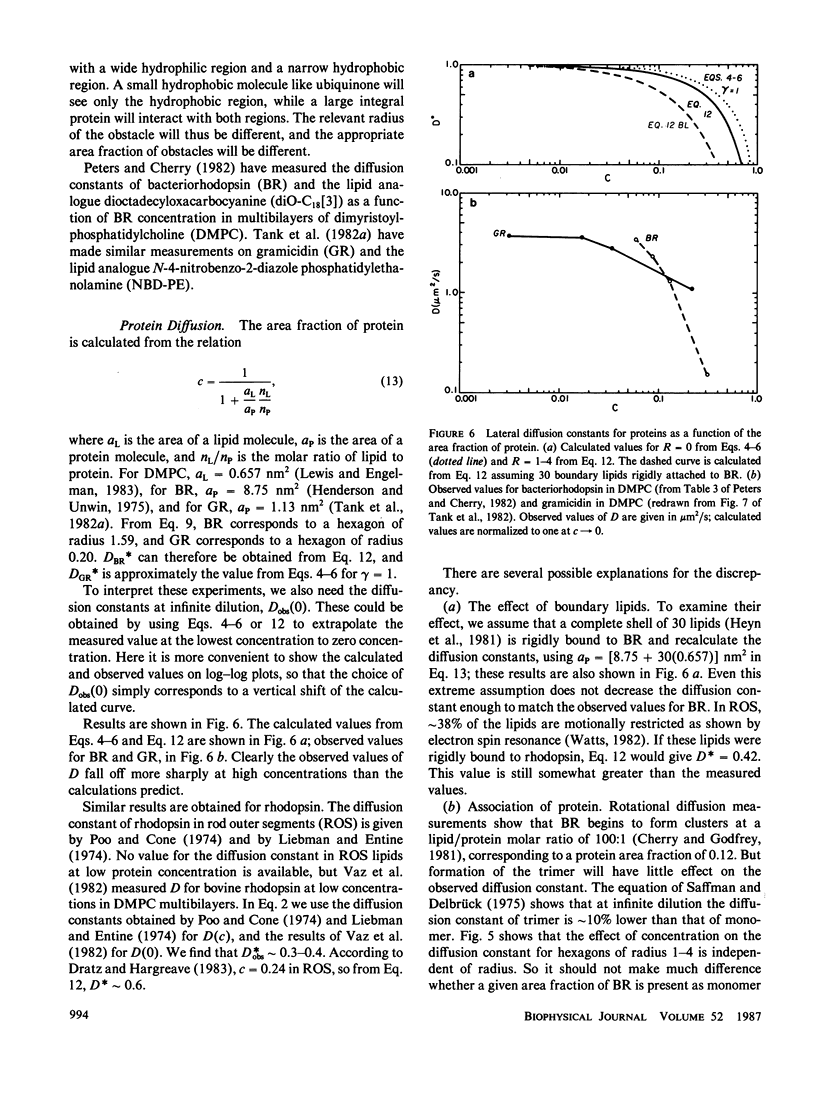
Lateral diffusion of mobile proteins and lipids (tracers) in a membrane is hindered by the presence of proteins (obstacles) in the membrane. If the obstacles are immobile, their effect may be described by percolation theory, which states that the long-range diffusion constant of the tracers goes to zero when the area fraction of obstacles is greater than the percolation threshold. If the obstacles are themselves mobile, the diffusion constant of the tracers depends on the area fraction of obstacles and the relative jump rate of tracers and obstacles. This paper presents Monte Carlo calculations of diffusion constants on square and triangular lattices as a function of the concentration of obstacles and the relative jump rate. The diffusion constant for particles of various sizes is also obtained. Calculated values of the concentration-dependent diffusion constant are compared with observed values for gramicidin and bacteriorhodopsin. The effect of the proteins as inert obstacles is significant, but other factors, such as protein-protein interactions and perturbation of lipid viscosity by proteins, are of comparable importance. Potential applications include the diffusion of proteins at high concentrations (such as rhodopsin in rod outer segments), the modulation of diffusion by release of membrane proteins from cytoskeletal attachment, and the diffusion of mobile redox carriers in mitochondria, chloroplasts, and endoplasmic reticulum.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D. Lateral motion of membrane proteins and biological function. J Membr Biol. 1983;75(1):1–10. doi: 10.1007/BF01870794. [DOI] [PubMed] [Google Scholar]
- Braun J., Abney J. R., Owicki J. C. How a gap junction maintains its structure. 1984 Jul 26-Aug 1Nature. 310(5975):316–318. doi: 10.1038/310316a0. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Godfrey R. E. Anisotropic rotation of bacteriorhodopsin in lipid membranes. Comparison of theory with experiment. Biophys J. 1981 Oct;36(1):257–276. doi: 10.1016/S0006-3495(81)84727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger J., Flores J., Petersen W. P. A milling crowd model for local and long-range obstructed lateral diffusion. Mobility of excimeric probes in the membrane of intact erythrocytes. Biophys J. 1986 May;49(5):987–1001. doi: 10.1016/S0006-3495(86)83727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson E. L., Reidler J. A. Analysis of cell surface interactions by measurements of lateral mobility. J Supramol Struct. 1979;12(4):481–489. doi: 10.1002/jss.400120408. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Blume A., Rehorek M., Dencher N. A. Calorimetric and fluorescence depolarization studies on the lipid phase transition of bacteriorhodopsin--dimyristoylphosphatidylcholine vesicles. Biochemistry. 1981 Dec 8;20(25):7109–7115. doi: 10.1021/bi00528a009. [DOI] [PubMed] [Google Scholar]
- Hughes B. D., Pailthorpe B. A., White L. R., Sawyer W. H. Extraction of membrane microviscosity from translational and rotational diffusion coefficients. Biophys J. 1982 Mar;37(3):673–676. [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Hou Y., Derzko Z., Wojcieszyn J., Organisciak D. Lipid lateral diffusion in the surface membrane of cells and in multibilayers formed from plasma membrane lipids. Biochemistry. 1981 Sep 1;20(18):5268–5275. doi: 10.1021/bi00521a027. [DOI] [PubMed] [Google Scholar]
- Jähnig F. No need for a new membrane model. Nature. 1981 Feb 19;289(5799):694–696. doi: 10.1038/289694a0. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Matrix control of protein diffusion in biological membranes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3576–3580. doi: 10.1073/pnas.78.6.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983 May 15;166(2):211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Lateral diffusion of visual pigment in photorecptor disk membranes. Science. 1974 Aug 2;185(4149):457–459. doi: 10.1126/science.185.4149.457. [DOI] [PubMed] [Google Scholar]
- McCloskey M., Poo M. M. Protein diffusion in cell membranes: some biological implications. Int Rev Cytol. 1984;87:19–81. doi: 10.1016/s0074-7696(08)62439-0. [DOI] [PubMed] [Google Scholar]
- Onoda GY, Liniger EG. Experimental determination of the random-parking limit in two dimensions. Phys Rev A Gen Phys. 1986 Jan;33(1):715–716. doi: 10.1103/physreva.33.715. [DOI] [PubMed] [Google Scholar]
- Pearson L. T., Chan S. I., Lewis B. A., Engelman D. M. Pair distribution functions of bacteriorhodopsin and rhodopsin in model bilayers. Biophys J. 1983 Aug;43(2):167–174. doi: 10.1016/S0006-3495(83)84337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Cherry R. J. Lateral and rotational diffusion of bacteriorhodopsin in lipid bilayers: experimental test of the Saffman-Delbrück equations. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4317–4321. doi: 10.1073/pnas.79.14.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Effects of impermeable patches on diffusion in a cell membrane. Biophys J. 1982 Aug;39(2):165–173. doi: 10.1016/S0006-3495(82)84504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Osborn M. J., Koppel D. E. Lateral mobility in reconstituted membranes--comparisons with diffusion in polymers. Nature. 1980 Jan 24;283(5745):346–350. doi: 10.1038/283346a0. [DOI] [PubMed] [Google Scholar]
- Sowers A. E., Hackenbrock C. R. Variation in protein lateral diffusion coefficients is related to variation in protein concentration found in mitochondrial inner membranes. Biochim Biophys Acta. 1985 Nov 21;821(1):85–90. doi: 10.1016/0005-2736(85)90157-9. [DOI] [PubMed] [Google Scholar]
- Tahir-Kheli RA, El-Meshad N. Correlated diffusion in two-dimensional systems. Phys Rev B Condens Matter. 1985 Nov 15;32(10):6166–6175. doi: 10.1103/physrevb.32.6166. [DOI] [PubMed] [Google Scholar]
- Tank D. W., Wu E. S., Meers P. R., Webb W. W. Lateral diffusion of gramicidin C in phospholipid multibilayers. Effects of cholesterol and high gramicidin concentration. Biophys J. 1982 Nov;40(2):129–135. doi: 10.1016/S0006-3495(82)84467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank D. W., Wu E. S., Webb W. W. Enhanced molecular diffusibility in muscle membrane blebs: release of lateral constraints. J Cell Biol. 1982 Jan;92(1):207–212. doi: 10.1083/jcb.92.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Criado M., Madeira V. M., Schoellmann G., Jovin T. M. Size dependence of the translational diffusion of large integral membrane proteins in liquid-crystalline phase lipid bilayers. A study using fluorescence recovery after photobleaching. Biochemistry. 1982 Oct 26;21(22):5608–5612. doi: 10.1021/bi00265a034. [DOI] [PubMed] [Google Scholar]
- Webb W. W., Barak L. S., Tank D. W., Wu E. S. Molecular mobility on the cell surface. Biochem Soc Symp. 1981;(46):191–205. [PubMed] [Google Scholar]
- Wu E. S., Tank D. W., Webb W. W. Unconstrained lateral diffusion of concanavalin A receptors on bulbous lymphocytes. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4962–4966. doi: 10.1073/pnas.79.16.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijeren H, Kutner R. Mean square displacement of a tracer particle in a hard-core lattice gas. Phys Rev Lett. 1985 Jul 8;55(2):238–241. doi: 10.1103/PhysRevLett.55.238. [DOI] [PubMed] [Google Scholar]


