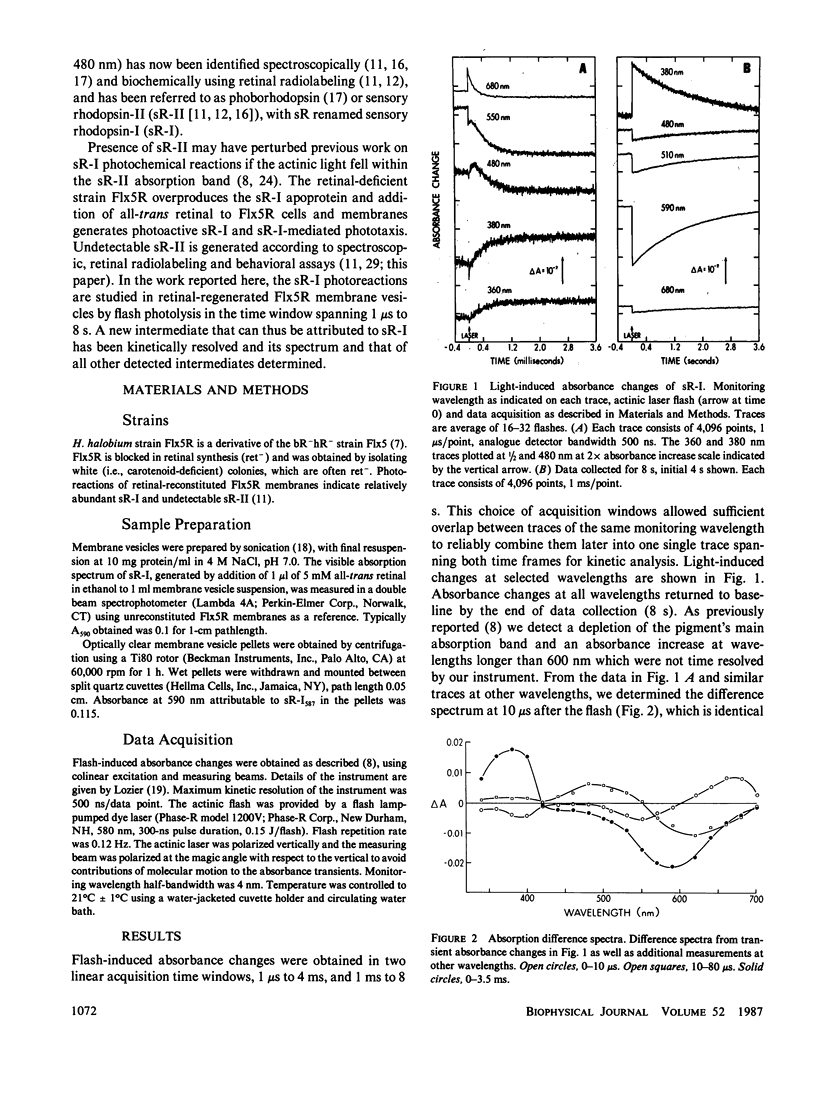
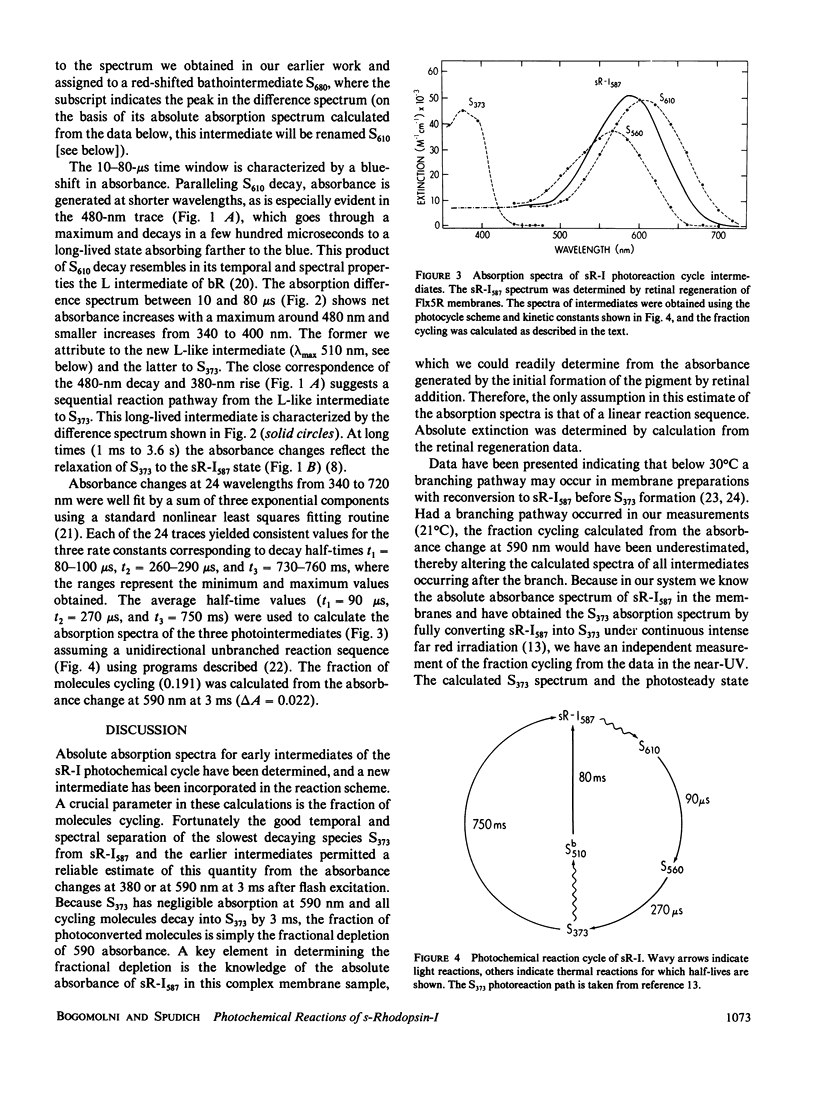
Abstract
Halobacterium halobium Flx mutants are deficient in bacteriorhodopsin (bR) and halorhodopsin (hR). Such strains are phototactic and the light signal detectors are two additional retinal pigments, sensory rhodopsins I and II (sR-I and sR-II), which absorb maximally at 587 and 480 nm, respectively. A retinal-deficient Flx mutant, Flx5R, overproduces sR-I-opsin and does not show any photochemical activity other than that of sR-I after the pigment is regenerated by addition of all-trans retinal. Using native membrane vesicles from this strain, we have resolved a new photointermediate in the sR-I photocycle between the early bathointermediate S610 and the later intermediate S373. The new form, S560, resembles the L intermediate of bR in its position in the photoreaction cycle, its relatively low extinction, and its moderate blue shift. It forms with a half-time of approximately 90 microseconds at 21 degrees C, concomitant with the decay of S610. Its decay with a half-time of 270 microseconds parallels the appearance of S373. From a data set consisting of laser flash-induced absorbance changes (300 ns, 580-nm excitation) measured at 24 wavelengths from 340 to 720 nm in a time window spanning 1 microsecond to 8 s we have calculated the spectra of the photocycle intermediates assuming a unidirectional, unbranched reaction scheme.
Full text
PDF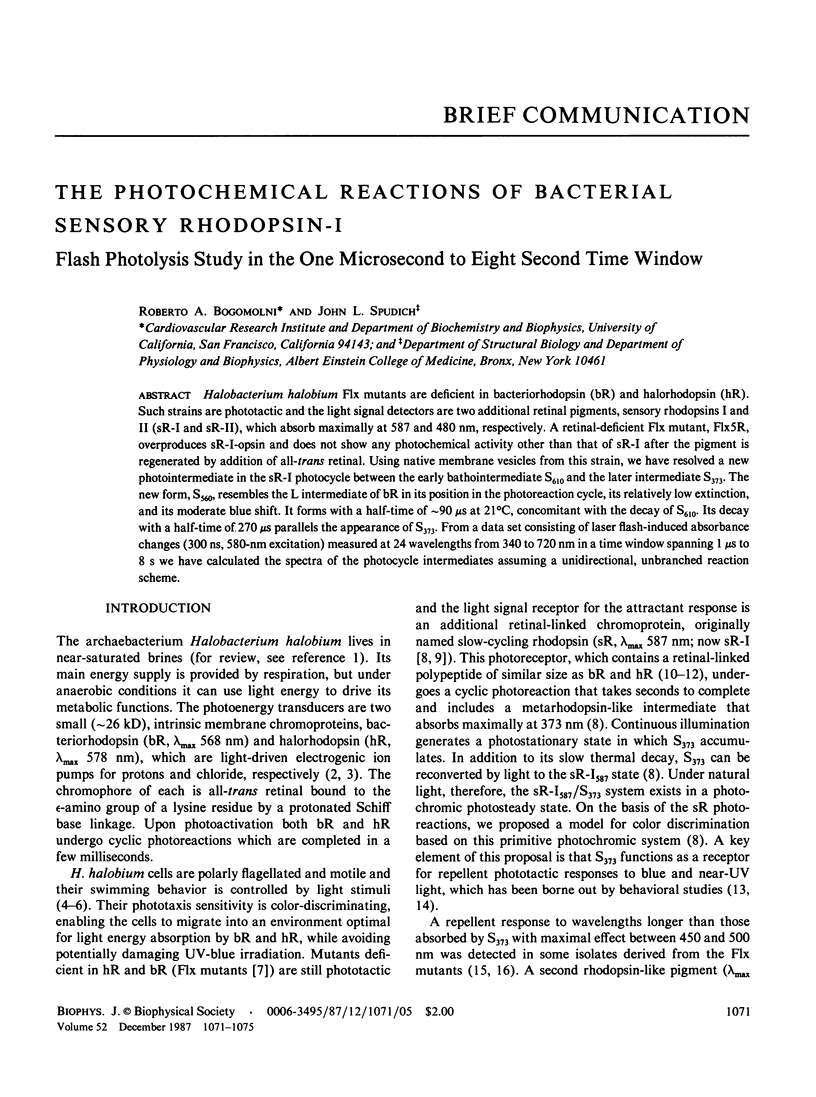


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogomolni R. A., Spudich J. L. Identification of a third rhodopsin-like pigment in phototactic Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6250–6254. doi: 10.1073/pnas.79.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabre M. Trigger and amplification mechanisms in visual phototransduction. Annu Rev Biophys Biophys Chem. 1985;14:331–360. doi: 10.1146/annurev.bb.14.060185.001555. [DOI] [PubMed] [Google Scholar]
- Hazemoto N., Kamo N., Terayama Y., Kobatake Y., Tsuda M. Photochemistry of two rhodopsinlike pigments in bacteriorhodopsin-free mutant of Halobacterium halobium. Biophys J. 1983 Oct;44(1):59–64. doi: 10.1016/S0006-3495(83)84277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgerson S. L., Mathew M. K., Bivin D. B., Wolber P. K., Heinz E., Stoeckenius W. Coupling between the bacteriorhodopsin photocycle and the protonmotive force in Halobacterium halobium cell envelope vesicles. III. Time-resolved increase in the transmembrane electric potential and modeling of the associated ion fluxes. Biophys J. 1985 Nov;48(5):709–719. doi: 10.1016/S0006-3495(85)83829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig B., Ebrey T., Callender R. H., Dinur U., Ottolenghi M. Photoisomerization, energy storage, and charge separation: a model for light energy transduction in visual pigments and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2503–2507. doi: 10.1073/pnas.76.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Halorhodopsin: a light-driven chloride ion pump. Annu Rev Biophys Biophys Chem. 1986;15:11–28. doi: 10.1146/annurev.bb.15.060186.000303. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., MacDonald R. E. Light-induced transport in Halobacterium halobium. Methods Enzymol. 1979;56:398–407. doi: 10.1016/0076-6879(79)56038-8. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain D. A., Amici L. A., Spudich J. L. Kinetically resolved states of the Halobacterium halobium flagellar motor switch and modulation of the switch by sensory rhodopsin I. J Bacteriol. 1987 Oct;169(10):4750–4758. doi: 10.1128/jb.169.10.4750-4758.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Parodi L. A., Lozier R. H. Procedure for testing kinetic models of the photocycle of bacteriorhodopsin. Biophys J. 1982 May;38(2):161–174. doi: 10.1016/S0006-3495(82)84543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer P., McGinnis K., Bogomolni R. A. Biochemical and spectroscopic characterization of the blue-green photoreceptor in Halobacterium halobium. Proc Natl Acad Sci U S A. 1987 Jan;84(2):402–406. doi: 10.1073/pnas.84.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich E. N., Bogomolni R. A., Spudich J. L. Genetic and biochemical resolution of the chromophoric polypeptide of halorhodopsin. Biochem Biophys Res Commun. 1983 Apr 15;112(1):332–338. doi: 10.1016/0006-291x(83)91835-1. [DOI] [PubMed] [Google Scholar]
- Spudich E. N., Spudich J. L. Control of transmembrane ion fluxes to select halorhodopsin-deficient and other energy-transduction mutants of Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4308–4312. doi: 10.1073/pnas.79.14.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich E. N., Sundberg S. A., Manor D., Spudich J. L. Properties of a second sensory receptor protein in Halobacterium halobium phototaxis. Proteins. 1986 Nov;1(3):239–246. doi: 10.1002/prot.340010306. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Mechanism of colour discrimination by a bacterial sensory rhodopsin. Nature. 1984 Dec 6;312(5994):509–513. doi: 10.1038/312509a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Spectroscopic discrimination of the three rhodopsinlike pigments in Halobacterium halobium membranes. Biophys J. 1983 Aug;43(2):243–246. doi: 10.1016/S0006-3495(83)84345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Mochizuki Y., Kamo N., Kobatake Y. Evidence that the long-lifetime photointermediate of s-rhodopsin is a receptor for negative phototaxis in Halobacterium halobium. Biochem Biophys Res Commun. 1985 Feb 28;127(1):99–105. doi: 10.1016/s0006-291x(85)80131-5. [DOI] [PubMed] [Google Scholar]
- Tomioka H., Takahashi T., Kamo N., Kobatake Y. Flash spectrophotometric identification of a fourth rhodopsin-like pigment in Halobacterium halobium. Biochem Biophys Res Commun. 1986 Sep 14;139(2):389–395. doi: 10.1016/s0006-291x(86)80003-1. [DOI] [PubMed] [Google Scholar]
- Wolff E. K., Bogomolni R. A., Scherrer P., Hess B., Stoeckenius W. Color discrimination in halobacteria: spectroscopic characterization of a second sensory receptor covering the blue-green region of the spectrum. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7272–7276. doi: 10.1073/pnas.83.19.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]



