Abstract
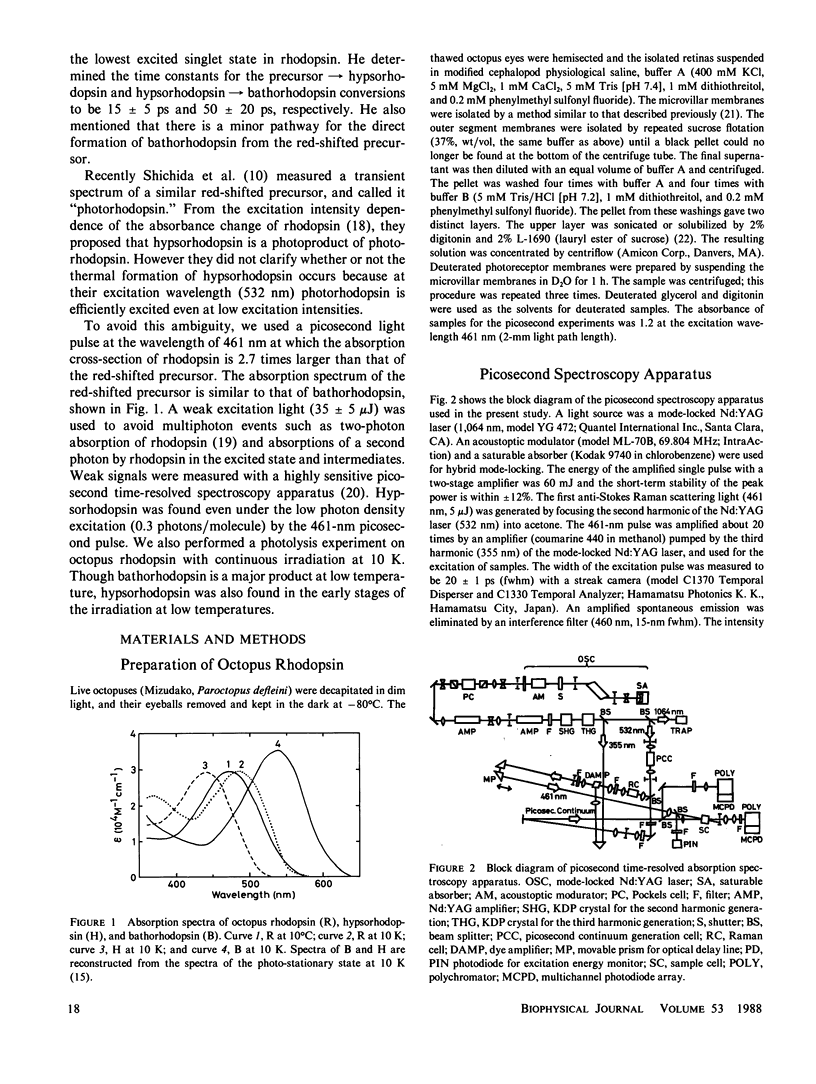
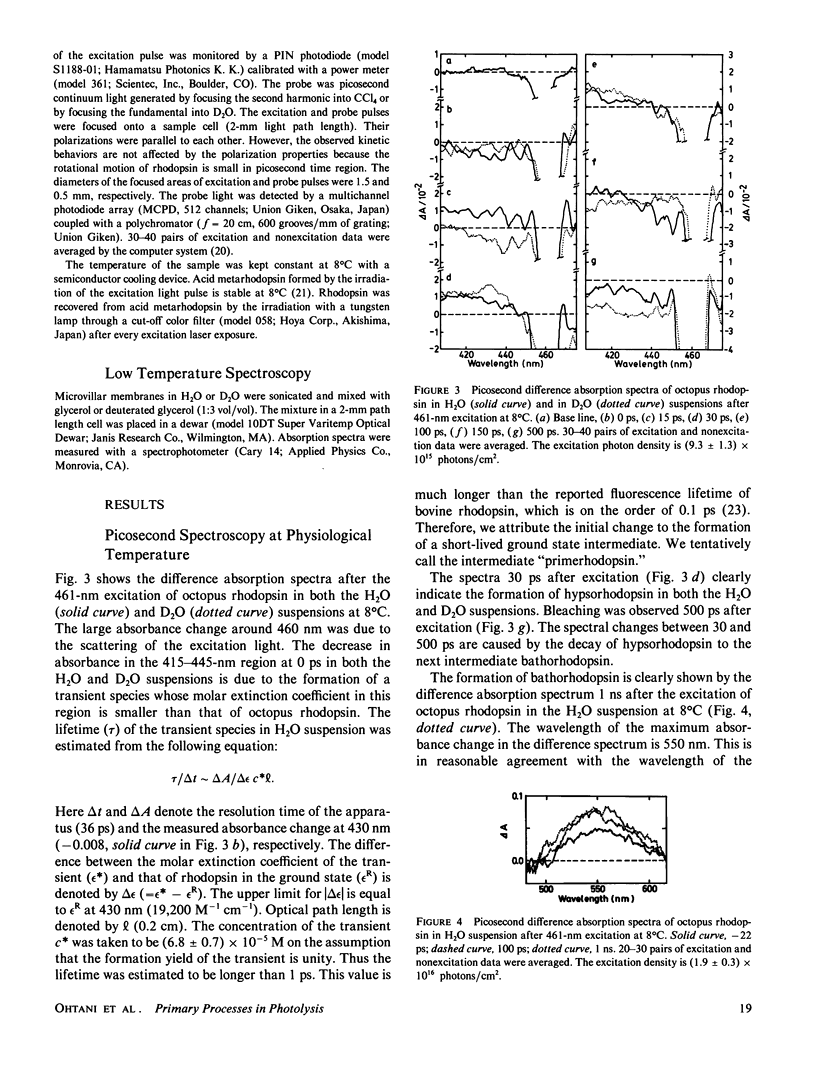
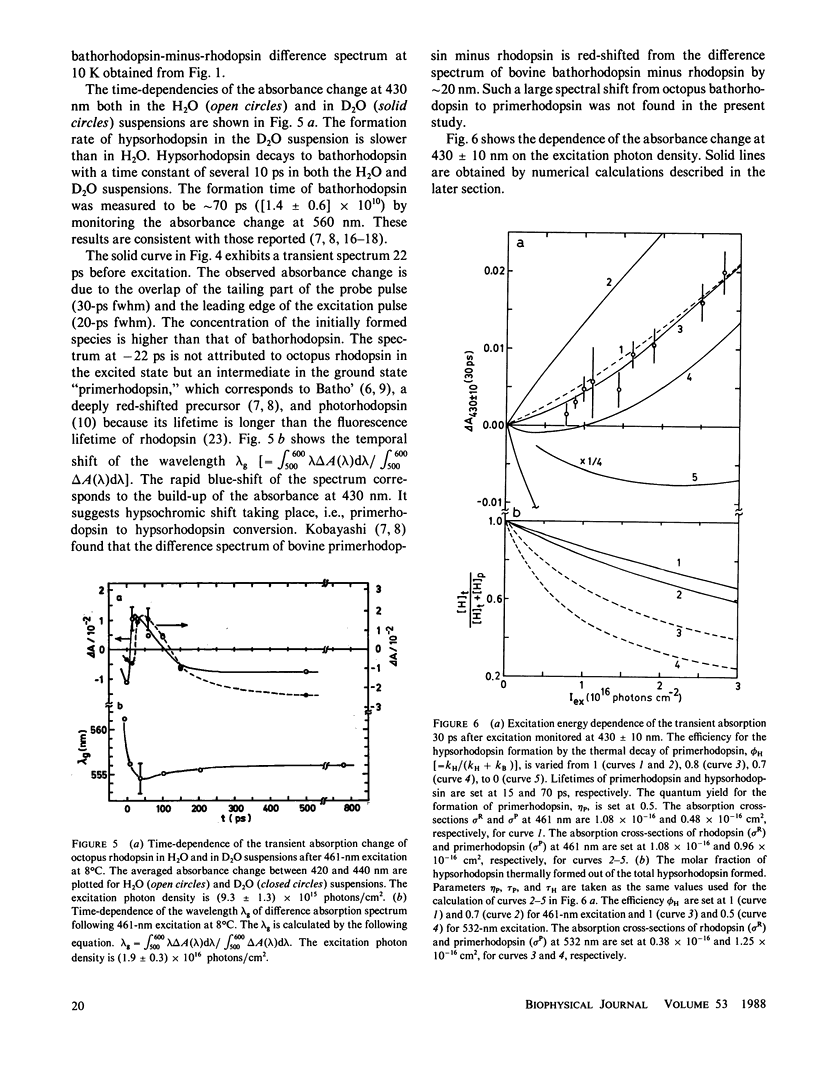
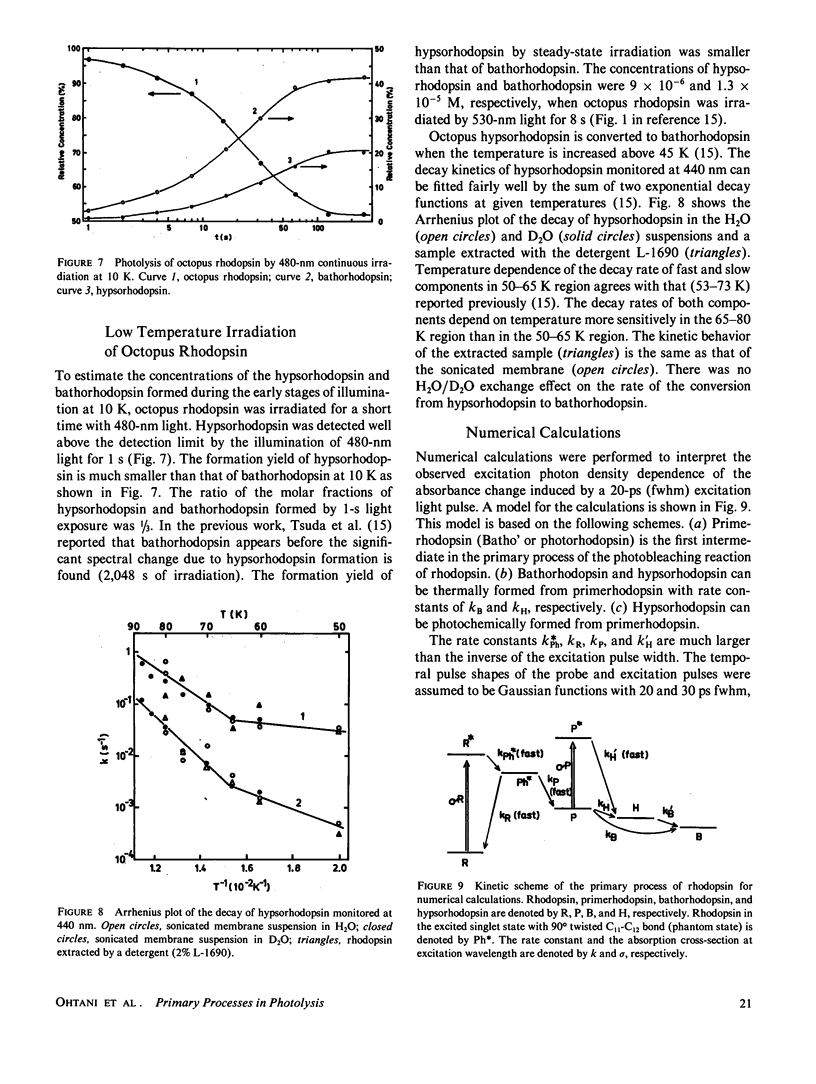
The photolysis of octopus rhodopsin was studied by picosecond time-resolved spectroscopy at physiological temperature (8°C) and by steady-state spectroscopy at very low temperature (10 K). Both hypsorhodopsin and bathorhodopsin were formed from a bathorhodopsin-like red-shifted intermediate “primerhodopsin,” which was the primary photoproduct with our time resolution (36 ps). Though it was proposed that hypsorhodopsin is formed solely by a multiphoton process, the present results obtained by using blue light pulses (461 nm) of low intensity showed that hypsorhodopsin is formed by a single photon mechanism via thermal decay from primerhodopsin. When the excitation intensity is increased, a channel for the photochemical formation of hypsorhodopsin from primerhodopsin is opened. There are two thermal pathways leading from primerhodopsin. One process is the formation of hypsorhodopsin, which is later thermally converted to bathorhodopsin, and the other is the direct formation of bathorhodopsin from primerhodopsin. The formation efficiencies at room temperature of hypsorhodopsin and bathorhodopsin at very low excitation intensity were estimated to be larger than 0.6 and smaller than 0.4, respectively. The formation of hypsorhodopsin was also found in the early stages of the irradiation of octopus rhodopsin with weak continuous light at 10 K. However bathorhodopsin is formed three times more efficiently than hypsorhodopsin at 10 K.
At physiological temperatures the formation of hypsorhodopsin in D2O takes place more slowly than in H2O. This indicates that the lifetime of primerhodopsin is decreased by H2O/D2O exchange. The rate constant for the primerhodopsin → bathorhodopsin conversion is more sensitive than that for the primerhodopsin → hypsorhodopsin conversion. The transformation of hypsorhodopsin to bathorhodopsin shows no deuterium effect at low temperature.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birge R. R., Murray L. P., Pierce B. M., Akita H., Balogh-Nair V., Findsen L. A., Nakanishi K. Two-photon spectroscopy of locked-11-cis-rhodopsin: evidence for a protonated Schiff base in a neutral protein binding site. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4117–4121. doi: 10.1073/pnas.82.12.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch G. E., Applebury M. L., Lamola A. A., Rentzepis P. M. Formation and decay of prelumirhodopsin at room temperatures. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2802–2806. doi: 10.1073/pnas.69.10.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukas A. G., Junnarkar M. R., Alfano R. R., Callender R. H., Kakitani T., Honig B. Fluorescence quantum yield of visual pigments: evidence for subpicosecond isomerization rates. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4790–4794. doi: 10.1073/pnas.81.15.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. H., Monger T. G., Alfano R. R., Aton B., Callender R. H. Cis-trans isomerisation in rhodopsin occurs in picoseconds. Nature. 1977 Sep 8;269(5624):179–180. doi: 10.1038/269179a0. [DOI] [PubMed] [Google Scholar]
- Honig B., Ebrey T., Callender R. H., Dinur U., Ottolenghi M. Photoisomerization, energy storage, and charge separation: a model for light energy transduction in visual pigments and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2503–2507. doi: 10.1073/pnas.76.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R., Kropf A. THE ACTION OF LIGHT ON RHODOPSIN. Proc Natl Acad Sci U S A. 1958 Feb;44(2):130–139. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G., Honig B., Ottolenghi M. Temperature and wavelength effects on the photochemistry of rhodopsin, isorhodopsin, bacteriorhodopsin and their photoproducts. Nature. 1977 Dec 8;270(5637):540–542. doi: 10.1038/270540a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi T. Existence of hypsorhodopsin as the first intermediate in the primary photochemical process of cattle rhodopsin. Photochem Photobiol. 1980 Aug;32(2):207–215. doi: 10.1111/j.1751-1097.1980.tb04011.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi T. Hypsorhodopsin: the first intermediate of the photochemical process in vision. FEBS Lett. 1979 Oct 15;106(2):313–316. doi: 10.1016/0014-5793(79)80522-0. [DOI] [PubMed] [Google Scholar]
- Matuoka S., Shichida Y., Yoshizawa T. Formation of hypsorhodopsin at room temperature by picosecond green pulse. Biochim Biophys Acta. 1984 Apr 26;765(1):38–42. doi: 10.1016/0005-2728(84)90154-3. [DOI] [PubMed] [Google Scholar]
- Nashima K., Mitsudo M., Kito Y. Studies on cephalopod rhodopsin. Fatty acid esters of sucrose as effective detergents. Biochim Biophys Acta. 1978 Sep 26;536(1):78–87. doi: 10.1016/0005-2795(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Pande A. J., Callender R. H., Ebrey T. G., Tsuda M. Resonance Raman study of the primary photochemistry of visual pigments. Hypsorhodopsin. Biophys J. 1984 Mar;45(3):573–576. doi: 10.1016/S0006-3495(84)84194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K., Applebury M. L., Rentzepis P. M. Primary photochemical event in vision: proton translocation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3119–3123. doi: 10.1073/pnas.74.8.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichida Y., Yoshizawa T. Squid hypsorhodopsin and bathorhodopsin by a picosecond laser photolysis. FEBS Lett. 1977 Aug 1;80(1):214–216. doi: 10.1016/0014-5793(77)80442-0. [DOI] [PubMed] [Google Scholar]
- Sundstrom V., Rentzepis P. M., Peters K., Applebury M. L. Kinetics of rhodopsin at room temperature measured by picosecond spectroscopy. Nature. 1977 Jun 16;267(5612):645–646. doi: 10.1038/267645a0. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Tokunaga F., Ebrey T. G., Yue K. T., Marque J., Eisenstein L. Behaviour of octopus rhodopsin and its photoproducts at very low temperatures. Nature. 1980 Oct 2;287(5781):461–462. doi: 10.1038/287461a0. [DOI] [PubMed] [Google Scholar]
- Tsuda M. Transient spectra of intermediates in the photolytic sequence of octopus rhodopsin. Biochim Biophys Acta. 1979 Mar 15;545(3):537–546. doi: 10.1016/0005-2728(79)90162-2. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA T., KITO Y. Chemistry of the rhodopsin cycle. Nature. 1958 Dec 6;182(4649):1604–1605. doi: 10.1038/1821604a0. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA T., WALD G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963 Mar 30;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]


