Abstract
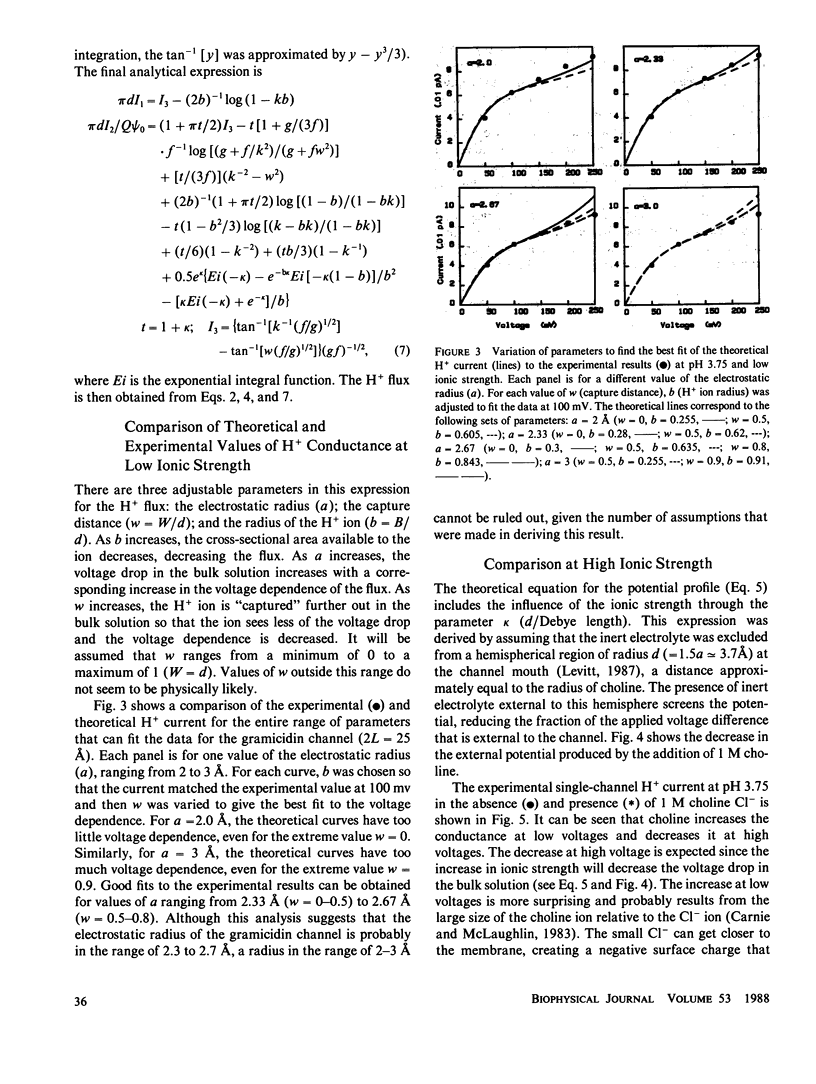
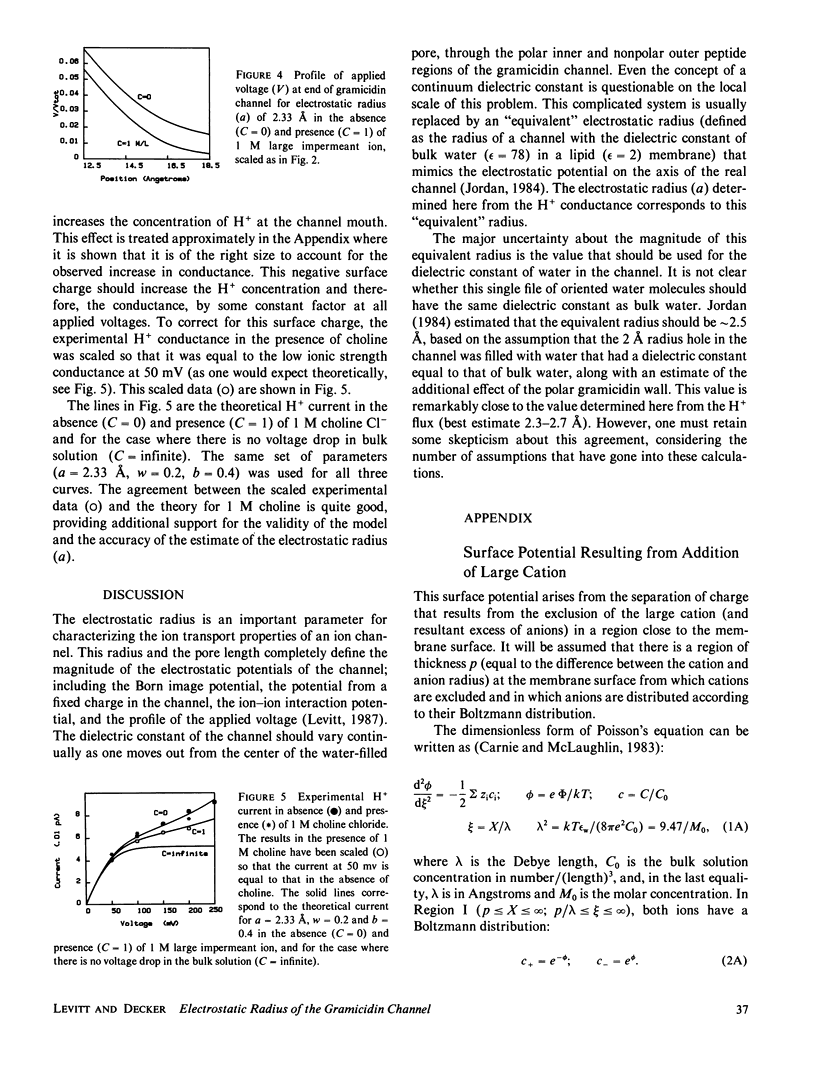
The results of Decker and Levitt (1987) suggest that the conductance of H+ ion through the gramicidin channel is limited primarily by diffusion in the bulk solution at the channel mouth. It is assumed in this paper that the H+ conductance is 100% diffusion limited. This means that all the factors that influence the H+ flux are external to the channel and are presumed to be known. In particular, the diffusion coefficient of H+ in this region is assumed to be equal to the bulk solution value and the only force acting on the ion is that due to the applied voltage. A model of the H+ flux is derived, based on the Nernst-Planck equation. It has three adjustable parameters: the electrostatic radius, the capture distance, and the radius of the H+ ion. The acceptable range of the parameters was determined by comparing the predictions of the model with the experimental measurements of the H+ conductance at pH 3.75. The best fit was obtained for an electrostatic radius in the range 2.3-2.7 A. This is in good agreement with earlier predictions (2.5 A) based on the assumption that the dielectric constant of the channel water is equal to that of bulk water. The addition of 1 M choline Cl- (an impermeant) increases the H+ current at low voltage and decreases it at high voltage. The increase can be explained by the small surface charge that results from the separation of charge produced by exclusion of the large choline cation (relative to Cl-) from the membrane surface. The decrease at high voltages can be accounted for by the change in the profile of the applied potential produced by the increase in ionic strength.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S. Ion movement through gramicidin A channels. Interfacial polarization effects on single-channel current measurements. Biophys J. 1983 Feb;41(2):135–146. doi: 10.1016/S0006-3495(83)84415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnie S., McLaughlin S. Large divalent cations and electrostatic potentials adjacent to membranes. A theoretical calculation. Biophys J. 1983 Dec;44(3):325–332. doi: 10.1016/S0006-3495(83)84306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker E. R., Levitt D. G. Use of weak acids to determine the bulk diffusion limitation of H+ ion conductance through the gramicidin channel. Biophys J. 1988 Jan;53(1):25–32. doi: 10.1016/S0006-3495(88)83062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth A. H., Hladky S. B. Effects of double-layer polarization on ion transport. Biophys J. 1987 Jan;51(1):27–36. doi: 10.1016/S0006-3495(87)83308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. C. Electrostatic modeling of ion pores. Energy barriers and electric field profiles. Biophys J. 1982 Aug;39(2):157–164. doi: 10.1016/S0006-3495(82)84503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G. Exact continuum solution for a channel that can be occupied by two ions. Biophys J. 1987 Sep;52(3):455–466. doi: 10.1016/S0006-3495(87)83234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G. Interpretation of biological ion channel flux data--reaction-rate versus continuum theory. Annu Rev Biophys Biophys Chem. 1986;15:29–57. doi: 10.1146/annurev.bb.15.060186.000333. [DOI] [PubMed] [Google Scholar]
- Levitt D. G. Strong electrolyte continuum theory solution for equilibrium profiles, diffusion limitation, and conductance in charged ion channels. Biophys J. 1985 Jul;48(1):19–31. doi: 10.1016/S0006-3495(85)83757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Diffusion-limited ion flow through pores. Biochim Biophys Acta. 1976 Dec 2;455(2):493–509. doi: 10.1016/0005-2736(76)90320-5. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos E. E., Brickmann J. Molecular dynamics of ion transport through transmembrane model channels. Annu Rev Biophys Biophys Chem. 1985;14:315–330. doi: 10.1146/annurev.bb.14.060185.001531. [DOI] [PubMed] [Google Scholar]