Abstract
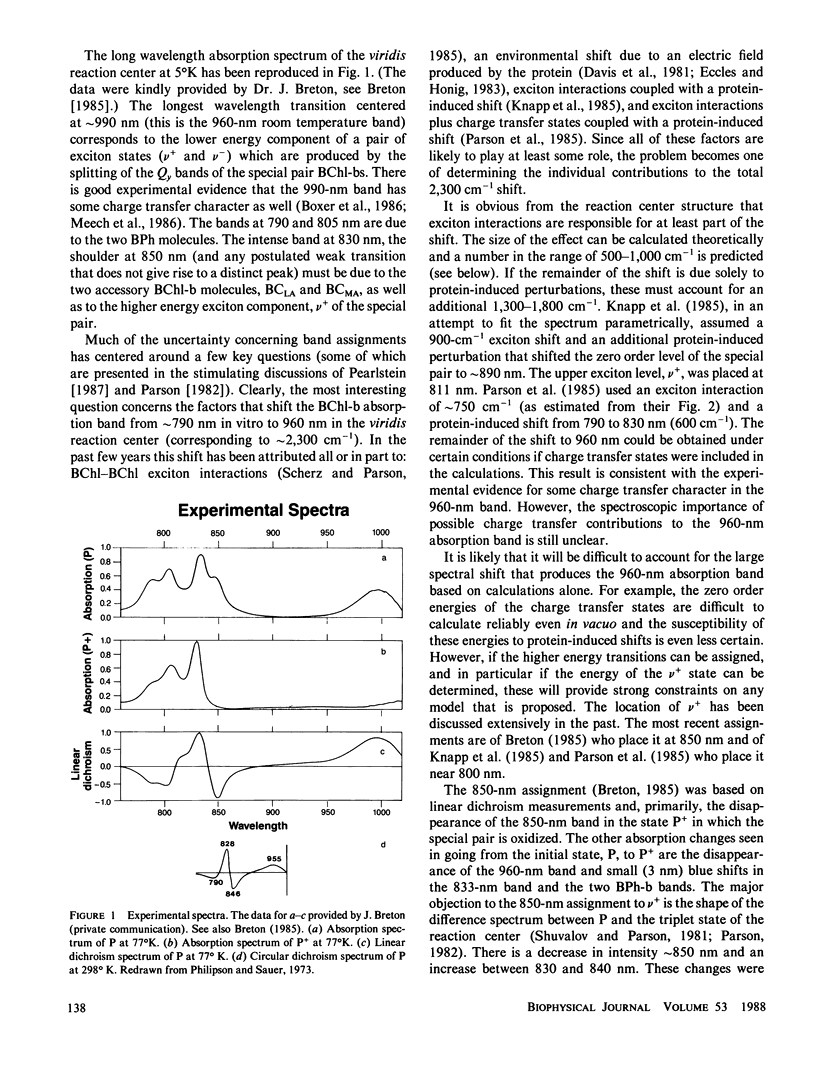
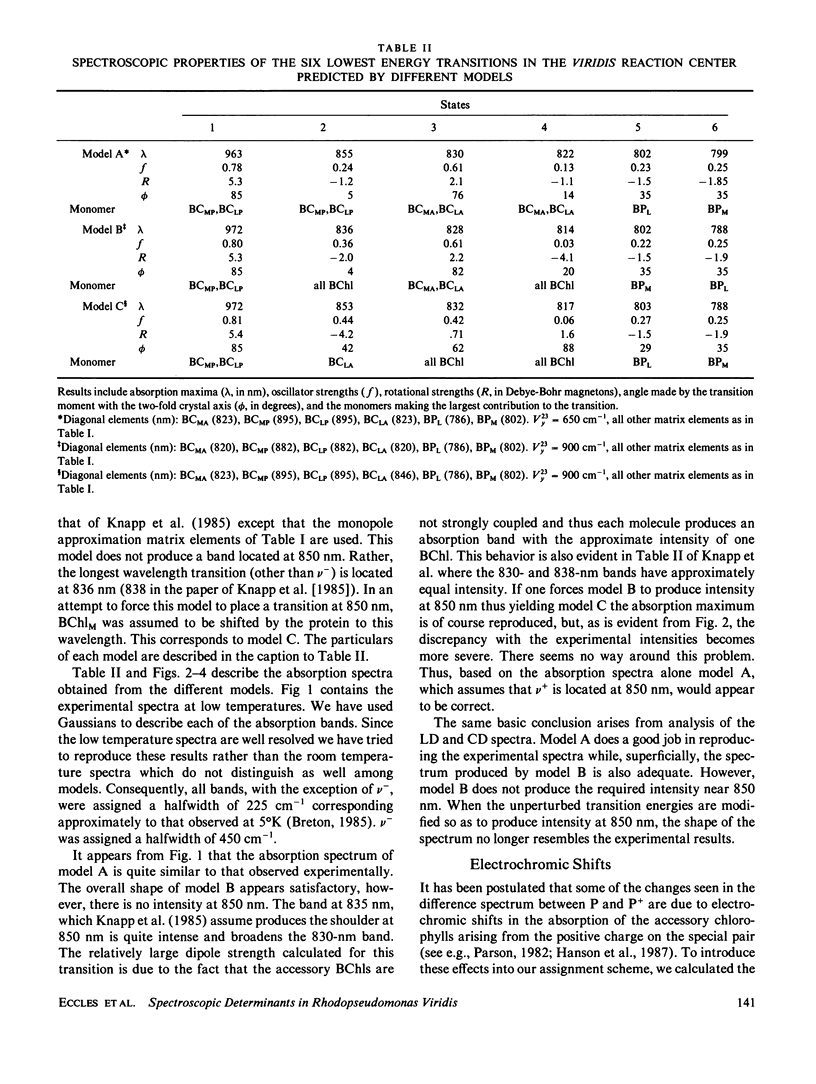
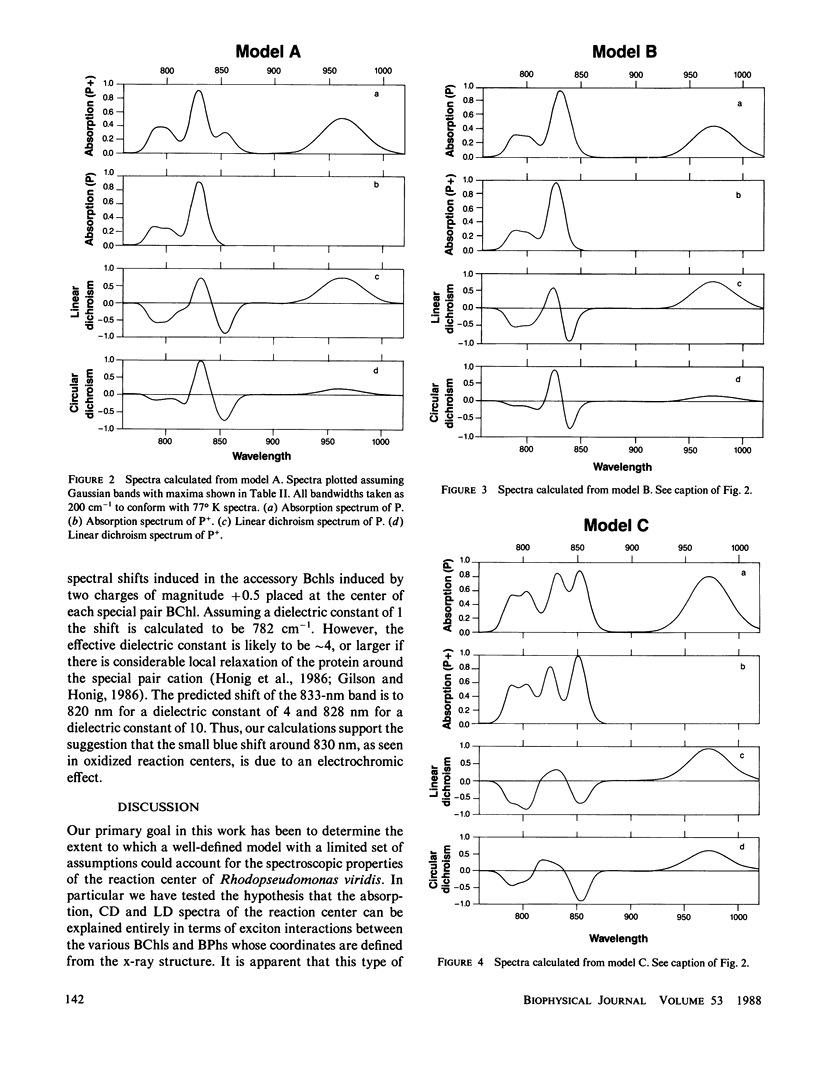
Assignments are proposed for the long wavelength absorption bands observed in the reaction center of Rhodopseudomonas viridis. The assignments are based on a theoretical treatment in which quantum mechanical calculations are first carried out on the individual chromophores of the reaction center. The energies and wave functions that are obtained are then introduced into an exciton-type perturbation treatment in which extensive configuration interaction is carried out between the excited states of the four bacteriochlorophylls and two bacteriopheophytins of the reaction center. Calculated values for absorption maxima, transition moments, linear dichroism, and rotational strength are compared with experiments in an attempt to distinguish among different assignments. The calculations alone do not lead to unambiguous assignments; indeed it is difficult to account for the reaction center spectra without introducing assumptions as to the effects of the protein on the energy levels of the individual molecules. Even if these effects are treated as free parameters, the experimental spectra still provide useful constraints that restrict the models that are possible. The major result of this work is that the weak 850-nm absorption band is due, primarily, to the higher energy exciton state of the bacteriochlorophyll special pair. Accounting for the 960-nm absorption band of the low energy exciton state of the special pair requires either that a large spectroscopic effect of the protein be introduced, or possibly, that charge transfer states play a major spectroscopic role. The difference in spectra seen in the formation of oxidized or triplet state reaction centers can be understood in terms of a combination of electrochromic effects and modified exciton interactions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Eccles J., Honig B. Charged amino acids as spectroscopic determinants for chlorophyll in vivo. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4959–4962. doi: 10.1073/pnas.80.16.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson M. K., Honig B. H. The dielectric constant of a folded protein. Biopolymers. 1986 Nov;25(11):2097–2119. doi: 10.1002/bip.360251106. [DOI] [PubMed] [Google Scholar]
- Honig B. H., Hubbell W. L., Flewelling R. F. Electrostatic interactions in membranes and proteins. Annu Rev Biophys Biophys Chem. 1986;15:163–193. doi: 10.1146/annurev.bb.15.060186.001115. [DOI] [PubMed] [Google Scholar]
- Knapp E. W., Fischer S. F., Zinth W., Sander M., Kaiser W., Deisenhofer J., Michel H. Analysis of optical spectra from single crystals of Rhodopseudomonas viridis reaction centers. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8463–8467. doi: 10.1073/pnas.82.24.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech S. R., Hoff A. J., Wiersma D. A. Role of charge-transfer states in bacterial photosynthesis. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9464–9468. doi: 10.1073/pnas.83.24.9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W. Photosynthetic bacterial reaction centers: interactions among the bacteriochlorophylls and bacteriopheophytins. Annu Rev Biophys Bioeng. 1982;11:57–80. doi: 10.1146/annurev.bb.11.060182.000421. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Sauer K. Comparative study of the circular dichroism spectra of reaction centers from several photosynthetic bacteria. Biochemistry. 1973 Jan 30;12(3):535–539. doi: 10.1021/bi00727a028. [DOI] [PubMed] [Google Scholar]


