Abstract
The functional activities of serum samples from human infants immunized with a glycoconjugate vaccine for Neisseria meningitidis serogroup C were assessed in a complement-mediated antibody-dependent serum bactericidal assay (SBA) and in a neonate rat model of protection from bacteremia. Selective serum samples from individual human infants were combined to make a panel of 11 serum pools to obtain a sufficient volume for testing. Each pool was assayed (i) for the anti-N. meningitidis serogroup C capsular polysaccharide (PS) immunoglobulin G (IgG) concentration as determined by reactivity in a direct-binding enzyme-linked immunosorbent assay, (ii) for bactericidal activity against N. meningitidis serogroup C strain C11, and (iii) for the ability to reduce bacteremia after passive transfer into a neonate rat model. Representative serum samples from infants who were not previously immunized with any N. meningitidis serogroup C vaccine served as a negative control. The prepared serum pools ranged in antibody concentration from 0.18 to 17.31 μg of IgG specific for N. meningitidis serogroup C PS per ml. For this serum panel, a direct relationship between concentrations of anti-N. meningitidis serogroup C PS-specific IgG and serum SBA titers (r = 0.9960) was observed. Passive transfer to neonate rats demonstrated the ability of postimmunization serum samples to significantly reduce (≥2-log10 reduction compared to control animals) the level of bacteremia following a challenge. Of 79 neonate rats that received ≥0.031 μg of human infant anti-N. meningitidis serogroup C PS IgG, 75 (94.9%) had a ≥2-log10 reduction in bacteremia, whereas of the animals that received <0.031 μg of antigen-specific IgG, 10.3% (4 of 39 rats) showed a ≥2-log10 reduction in bacteremia. It was concluded that the anti-N. meningitidis serogroup C PS IgG antibody induced by this glycoconjugate vaccine had in vitro functional activity (as determined by a SBA) and also afforded protection against meningococcal bacteremia in an animal model.
Glycoconjugate vaccines for Neisseria meningitidis serogroup C have been proven to be immunogenic and well tolerated in clinical trials (1, 4-9, 11, 12, 25-28, 36-38, 49). Additionally, a recently licensed N. meningitidis serogroup C glycoconjugate (MnCC) vaccine (Meningitec; Wyeth Vaccines, Pearl River, N.Y.) has demonstrated efficiency in reducing invasive disease in the United Kingdom since its introduction in 1999 (36). As prelicensure efficacy trials appear to be unfeasible to implement because of the unpredictable and sporadic nature of this disease, surrogate markers of efficacy aid in assessing new potential vaccines.
The serum bactericidal assay (SBA) has been the preferred method for determining the immune status with respect to N. meningitidis serogroup C. Goldschneider et al. (17) and others (34, 47, 48) have demonstrated that the presence of complement-mediated antibody-dependent serum bactericidal activity correlates with protection against naturally occurring meningococcal infection in humans. The higher frequency of recurrent meningococcal infections among individuals with terminal complement component (TCC) deficiencies (C5 to C9) suggests that bactericidal antibodies are important for protection (13, 30, 35, 39). In an attempt to substantiate data obtained from in vitro assays, researchers have employed animal models that mimic human disease.
Animal models of protection against pathogenic neisseriae have been described (10, 15, 16, 33, 43, 45), even though pathogenic neisseriae have a niche for a human host. The infant rat model has been previously used to evaluate monoclonal antibodies (18, 41, 43) and pooled hyperimmune human serum (22, 42) elicited by N. meningitidis serogroup B outer membrane protein vaccines. In the studies presented here, a neonate rat challenge model of bacteremia was chosen to evaluate the protective activity of serum samples from human infants who had received an MnCC vaccine. This report describes the relationships among the concentration of N. meningitidis serogroup C polysaccharide (PS)-specific immunoglobulin G (IgG), SBA titers, and the capacity of passively transferred sera to protect neonate rats from bacteremia due to N. meningitidis serogroup C.
MATERIALS AND METHODS
Human serum samples.
Serum samples were selected from groups of infants who were enrolled in a trial conducted by Vanderbilt University, Nashville, Tenn. (37). Serum samples were obtained from infants who were either immunized with three doses (2 or 10 μg) of an MnCC vaccine (Meningitec; Wyeth Vaccines) at 2, 4, and 6 months of age or from infants similar in age who did not receive the MnCC vaccine. The limited volume of sera obtained from human infants required the pooling of equal volumes from multiple specimens (n = 10) with similar antimeningococcal PS (anti-MnCPS) antibody concentrations. In order to assess potential protection thresholds in the infant rat challenge model, 11 distinct serum pools were made representing a range of anti-MnCPS IgG concentrations (Table 1). The concentrations of the pools were subsequently determined and found to range from 0.18 to 17.31 μg/ml (Table 1). In order to optimize the conditions of this neonate rat model, serum samples were obtained from adults 1 month after they had received a singe dose of a commercially available quadrivalent (A/C/Y/W-135) MnCPS vaccine (Menomune; Connaught Laboratories, Swiftwater, Pa.) in a trial conducted by Vanderbilt University (37) and from healthy adult volunteers. The adult immune serum samples were determined to have concentrations ranging from 1.65 to 45.5 μg of anti-MnCPS-specific antibody per ml (Table 2). Selected adult serum samples were assayed for antibody-dependent complement-mediated bactericidal activity in an SBA and for protective properties in an infant rat challenge model. Serum samples from healthy adults who did not receive an N. meningitidis serogroup C vaccine, ranging from undetectable quantities (<0.1 μg/ml) to 3.44 μg of anti-MnCPS-specific IgG antibody per ml, were included for comparison. All human serum samples were heat treated (56°C for 30 min) to inactivate complement prior to use in the SBA or neonate rat model.
TABLE 1.
Protective activity of anti-MnCPS IgG antibody from infants immunized with an MnCC vaccine in a neonate rat challenge model
| Serum poola | Pooled infant serum
|
Infant serum in rats
|
Reduction in bacteremia (log10)f | ||
|---|---|---|---|---|---|
| Concn of anti-MnCPS IgG (μg/ml)b | Bactericidal titerc | Total anti-MnCPS IgG (μg/rat)d | Bactericidal titer (calculated)e | ||
| A | 0.18 | <10 | 0.0009g | <0.05 | +0.31h |
| A | 0.18 | <10 | 0.0036 | <0.2 | 0.00 |
| B | 0.53 | 90 | 0.012 | 1.8 | 0.52 |
| C | 1.13 | 220 | 0.023 | 4.4 | 1.86 |
| D | 1.57 | 425 | 0.031 | 8.5 | 3.64i |
| E | 2.27 | 420 | 0.045 | 8.4 | 3.48i |
| F | 2.74 | 585 | 0.055 | 11.7 | 3.72i |
| G | 3.53 | 605 | 0.071 | 12.1 | 3.17i |
| H | 3.85 | 595 | 0.077 | 11.9 | 3.65i |
| I | 5.19 | 860 | 0.104 | 17.2 | 3.72i |
| J | 7.20 | 1,235 | 0.144 | 24.7 | 3.02i |
| K | 17.31 | 2,695 | 0.346 | 53.9 | 3.72i |
Pools were prepared from infants (n = 10) with similar anti-MnCPS IgG concentrations. A, serum from infants who did not receive an MnC vaccine; B to K, serum from infants who received an MnCC vaccine.
Anti-MnCPS IgG concentration in pooled human infant serum as determined by ELISA.
Values represent the reciprocal of the highest dilution of pooled infant serum that killed ≥50% of the N. meningitidis strain C11 target cells in an SBA (bactericidal titer).
Values represent amounts of anti-MnCPS-specific IgG passively transferred into rat pups 18 to 20 h prior to a challenge (unless otherwise noted, rat pups received 0.1 ml of a 1:5 dilution of pooled infant serum).
Calculated bactericidal titer of neonate rat serum after passive transfer (bactericidal titer of pooled human infant serum ÷ dilution factor).
Values represent the average log10 reduction in bacteremia in neonate rats 3 h after a challenge with N. meningitidis strain C11 compared to that in the negative control group.
Rat pups received 0.1 ml of a 1:20 dilution of serum pool A.
A positive value indicates an increase in bacteremia 3 h after a challenge.
Significantly different (P < 0.05 as determined by the Student t test) from values obtained from a group of normal rat pups that received 0.1 ml of a 1:5 dilution of serum pool A.
TABLE 2.
Protective effect of passively transferred adult serum in neonate rats challenged with group C N. meningitidis strain C11
| Seruma | Adult serum
|
Adult serum in rats
|
Reduction in bacteremia (log10)f | ||
|---|---|---|---|---|---|
| Concn of anti-MnCPS IgG (μg/ml)b | Bactericidal titerc | Total anti-MnCPS IgG (μg/rat)d | Bactericidal titer (calculated)e | ||
| 1g | 0.05 | 8 | 0.001 | 0.16 | 0.00 |
| 2 | 0.11 | 4 | 0.002 | 0.08 | 0.37 |
| 3 | 0.17 | <4 | 0.003 | <0.08 | 0.64 |
| 4 | 0.58 | 8 | 0.012 | 0.16 | 0.36 |
| 5 | 1.09 | 16 | 0.022 | 0.32 | 1.26 |
| 6 | 1.65 | 25 | 0.033 | 0.50 | 0.25 |
| 7 | 3.09 | 50 | 0.062 | 1.00 | 0.42 |
| 8 | 3.21 | 50 | 0.062 | 1.00 | 0.18 |
| 9 | 3.44 | 128 | 0.069 | 2.56 | 1.56 |
| 10 | 3.58 | 640 | 0.072 | 12.80 | 3.38h |
| 11 | 3.60 | 300 | 0.072 | 6.00 | 4.17h |
| 12 | 4.48 | 6,144 | 0.090 | 122.88 | 4.16h |
| 13 | 8.02 | 3,072 | 0.160 | 61.44 | 3.67h |
| 14 | 18.20 | 512 | 0.364 | 10.24 | 3.65h |
| 15 | 21.42 | 149 | 0.428 | 2.98 | 2.45h |
| 16 | 45.50 | 1,536 | 0.910 | 30.72 | 4.07h |
Individual serum samples from nonimmunized healthy adults (samples 1 to 5 and 9) and serum samples obtained from individuals who received one dose of a tetravalent meningococcal PS vaccine (A/C/Y/W-135) were tested.
Values represent concentrations of MnCPS-specific antibody in adult serum as determined by ELISA.
Values represent the reciprocal of the highest dilution of antiserum that killed ≥50% of the N. meningitidis strain C11 target cells in an SBA (bactericidal titer).
Amount of anti-MnCPS IgG passively transferred into rat pups (rat pups received 0.1 ml of a 1:5 dilution of individual serum) 18 to 20 h prior to a challenge with a rat-passaged isolate of group C N. meningitidis strain C11.
Calculated bactericidal titer of neonate rat serum after passive transfer (bactericidal titer of human adult sera ÷ serum dilution factor of 50).
Values represent the average log10 reduction in bacteremia in neonate rats 3 h after a challenge with group C N. meningitidis strain C11 compared to that of the negative control group that received 0.1 ml of a 1:5 dilution of human adult serum 1.
Designated negative control group for this experiment.
Significantly different (P < 0.05 as determined by the Student t test) from values obtained from a group of normal rat pups that received negative control antiserum.
Bacterial strain and growth conditions.
A rat-passaged isolate of N. meningitidis serogroup C strain C11 was used both as a target strain in the SBA and as the challenge strain in the neonate rat challenge model of bacteremia. The parental C11 strain was obtained from G. Carlone at the Centers for Disease Control and Prevention and was originally obtained by E. Gotschlich in 1965 (19). Parental strain C11 was grown on GCK agar plates (GC Agar; Difco Laboratories, Detroit Mich.) with Kellogg's supplement (24) (dextrose, 4 g/liter; glutamine, 0.1 g/liter; cocarboxylase, 0.2 mg/liter; ferric nitrate, 5.0 mg/liter) for approximately 18 h at 36°C in 5% CO2. The C11 parental strain was then diluted to a concentration of 107 CFU per ml in phosphate-buffered saline containing 0.5 mM magnesium chloride and 0.15 mM calcium chloride (PCM), and 0.1 ml was injected intraperitoneally (i.p.) into 3- to 4-day-old Sprague-Dawley rats. Three hours after i.p. injection with a challenge inoculum, rats were sacrificed by exposure to CO2 and bled by cardiac puncture. Aliquots of whole blood (40 μl) were then plated onto GCK agar plates and incubated at 36.5°C with 5% CO2 for at least 18 h. Colonies obtained from rat blood consistent with the phenotype of strain C11 were then subcultured on fresh GCK agar plates and incubated at 36.5°C with 5% CO2. Growth from subcultured plates was suspended in GC medium containing 20% sterile glycerol without Kellogg's supplement, aliquoted, frozen at −70°C, and designated rat-passaged N. meningitidis serogroup C strain C11. Rat-passaged frozen stock cultures were checked for purity with an additional subculture. Capsule expression of the rat-passaged subculture was verified by Western blot analysis with anti-MnCPS-specific polyclonal antiserum (data not shown).
For use in assays, rat-passaged C11 cells were grown overnight on GCK agar incubated at 36°C with 5% CO2. An additional subculture onto fresh GCK agar plates for an additional 3 to 4 h was not necessary. Preliminary studies indicated that rat-passaged C11 cells from plates incubated for 16 to 18 h yield concentrations of viable cells similar to that obtained from a 3- to 4-h subculture (data not shown). For the SBA, harvested cells were suspended in PCM to a concentration of approximately 1 × 105 to 3 × 105 (±500) CFU/ml. For use as a challenge strain in the neonate rat model, cells were suspended in PCM to a concentration of 1 × 106 to 3 × 106 CFU/ml within an hour of injection.
SBA.
The method used for the SBA was similar to that described by Mountzouros and Howell (32). The assay described here was performed with sterile 96-well assay plates. Target cells were prepared by diluting an overnight culture to a concentration of 1,000 to 3,000 CFU per 10 μl. Results of assays performed in our laboratory during the period of assay development indicated that either N. meningitidis serogroup C strain C11 or the rat-passaged strain could be used as a target strain in an SBA and generate similar results (data not shown). Reaction mixtures containing target cells (10 μl), test serum (5 μl undiluted or diluted), and PCM (25 μl) along with 10 μl of complement were incubated at 36°C in 5% CO2 for 30 min after slight agitation. Rabbit serum (lot 26517; Pel-Freez, Brown Deer, Wis.) found not to have significant bactericidal activity against the target cells was the source of the complement used in the SBA. Additional sets of wells were used to generate a standard curve. Standard curve wells (duplicate wells) included complement (10 μl), PCM (30 μl), and a suspension of target cells representing 0, 25, 50, 75, 90, and 100% of the target cells used in the assay (10 μl). After incubation, reactions were terminated by addition of 200 μl of an alamarBlue (Trek Diagnostic Systems, Westlake, Ohio) and molten agarose (melted and cooled to 42°C) additive consisting of a modified version of Frantz medium (14) (glutamic acid, 1.3 g/liter; cysteine, 0.02 g/liter; sodium phosphate dibasic heptahydrate, 10 g/liter; potassium chloride, 0.09 g/liter; sodium chloride, 6.0 g/liter; ammonium chloride, 1.25 g/liter; yeast extract dialysate, 40 ml/liter [final concentration, 0.2%, wt/vol]) containing 10 μl of alamarBlue and 0.7% (wt/vol) SeaPlaque low-melting-point agarose (FMC Bioproducts, Rockland, Maine). Reaction mixtures in wells of microtiter plates were allowed to solidify, covered with sterile plate covers, and incubated for 6 to 8 h at 36°C in 5% CO2.
In the fluorescence SBA, complement-mediated antibody-dependent bactericidal activity was determined by comparing the number of arbitrary fluorescence units (AFU) obtained from reaction wells containing test serum to the number of AFU obtained from standard curve wells containing 50% of the total number of target cells after incubation. SBA-determined bactericidal titers were expressed as the reciprocal of the highest dilution of test serum that yielded numbers of AFU lower than or equal to the numbers of AFU obtained in the standard curve wells containing 50% of the target cells (numbers of AFU correlate to the numbers of surviving bacteria [32]). Numbers of AFU obtained from control wells not containing viable cells were considered background fluorescence and were subtracted from numbers of AFU obtained in wells containing viable bacteria.
Instrumentation.
Fluorescence was detected with a Cytofluor 4000 microtiter plate reader (excitation wavelength of 530 nm and emission wavelength of 580 nm; Perceptive Biosystems, Framingham, Mass.). Numbers of AFU were determined by averaging the fluorescence in three consecutive measurements per well at a gain of approximately 50. Fluorescence was detected from the bottom of each reaction well of microtiter plates at specified time points (30-min intervals or between 360 and 480 min) after introduction of 200 μl of alamarBlue-agarose additive and incubation in the temperature-controlled chamber of the Cytofluor 4000 plate reader set at 36°C without CO2.
ELISA.
The ELISA method used to determine IgG antibodies reactive to MnCPS in human serum was previously described (46). Medium-binding polystyrene microtiter plates (Nalge Nunc International, Naperville, Ill.) were coated with an optimal concentration of highly purified capsular PS isolated from N. meningitidis serogroup C (Wyeth-Lederle Vaccines, Sanford, N.C.). Human serum, starting at a 50-fold dilution and serially diluted twofold, was added to wells of antigen-coated plates. Bound antibodies were detected by adding goat anti-human IgG-alkaline phosphatase labeled antibody, followed by p-nitrophenyl phosphate. Absorbances were determined at 405 nm with a 690-nm reference wavelength on a microtiter plate reader. A value was assigned to the specimen with a linear regression endpoint (0.3 absorbance unit) analysis comparing absorbances of the specimen versus dilutions of a reference serum (CDC 1992) containing 24.1 μg of IgG antibody per ml. The lower quantitation limit of the assay is 0.10 μg/ml. Values of less than 0.10 μg/ml are reported as 0.05 μg/ml.
Bacterial challenge.
The bacterial challenge inoculum used in this study was similar to that described by Nassif et al. (33). In this study, rat-passaged C11 cells were grown overnight on GCK agar incubated at 36.5°C with 5% CO2 and then suspended in PCM to an optical density corresponding to a concentration of 1 × 106 to 3 × 106 CFU/ml. Purity evaluation and enumeration of CFU in the challenge suspension were done by counting colonies from serial dilutions of challenge material that were plated on GCK agar plates incubated for at least 18 h at 36.5°C with 5% CO2.
Neonate rat challenge model and animal strain and age.
Sprague-Dawley rat pups (3 to 4 days old) were obtained from Taconic Labs (Germantown, N.Y.). Neonate rats birthed by experienced pregnant rats were shipped and housed in the same vivarium. Rat pups that are 4 to 5 days old weigh approximately 13 (±1) g, and the total blood volume of a healthy rat pup is approximately 7% of its body weight (approximately 0.9 ± 0.1 ml of whole blood per rat). Upon arrival, animals were observed for the purpose of recording numbers of animals and animal health. Only rats appearing in good health were used in this investigation. Prior to any manipulation, infant rats were marked for identification and then randomized into equal groups, approximately 10 pups per mother. Groups (n ≅ 10) of neonate rats were injected i.p. with 0.1 ml of pooled human infant serum or individual human adult serum samples diluted 1:5 (final dilution in rat serum = 1:50, except for one negative control group that resulted in a 1:200 dilution). After injection, each group of rat pups was returned to the cage containing the mother rat. Eighteen to 20 h after passive immunization, rat pups were injected i.p. with 0.1 ml of a bacterial suspension of a challenge inoculum containing approximately 5.0 log10 CFU. Preliminary studies demonstrated that in this animal challenge model, inocula containing ≥3 log10 CFU cause bacteremia in neonate rats in a dose-dependent manner, with death occurring in 50% of neonate rats receiving ∼7.0 log10 CFU (data not shown). Nonimmunized neonate rats receiving 5.0 log10 CFU of the rat-passaged challenge strain became bacteremic, with peak levels between 3 and 6 h and subsequently decreasing over a period of 48 h. In this study, the level of bacteremia 3 to 6 h after a challenge was determined to be 4.5 ± 0.5 log10 CFU/ml. Neonate rat pups were sacrificed 3 h after a challenge by exposure to CO2 and immediately bled by cardiac puncture. Whole blood and/or diluted blood (40 μl) was plated in duplicate onto GCK agar medium. Plated blood was incubated for 18 to 24 h at 36.5°C with 5% CO2. The number of colonies consistent in appearance with strain C11 on each inoculated GCK agar plate was determined. Numbers of colonies on plates from individual rat pup blood samples (ranging from 10 to 500 CFU) were averaged and multiplied by the dilution factor to determine the number of CFU per milliliter of blood.
In the absence of passively administered human serum, the lower limit of bacteremia detectable equaled one colony when plates were inoculated with 40 μl of undiluted blood (1 CFU/0.04 ml = 25 CFU/ml or 1.4 log10 CFU/ml of blood). When no colonies were recovered on plates, a value of 0.5 CFU (or 0.5 CFU/0.04 ml = 12.5 CFU/ml or 1.10 log10 CFU/ml of blood) was used. Protection was determined as the ability of passively transferred serum from immunized humans to reduce the level of bacteremia in neonate rats ≥2.0 log10 CFU/ml of blood (or ∼31,000 CFU/ml of blood) compared to the level in the group of rats receiving serum from humans who did not receive an N. meningitidis serogroup C vaccine.
Decomplementation of neonate rats with CVF.
Cobra venom factor (CVF) purified from Naja naja kaouthi (Venom Supplies, Tanunda, Australia) was used at a sublethal dose of 10 μg of CVF in 0.1 ml of phosphate-buffered saline per rat. CVF is a structural and functional analog of human complement factor 3 (C3). Thus, in the presence of factor B, factor D, and Mg2+, CVF can form a stable CVF-Bb complex, which is a C3-C5 convertase enzyme (a positive regulator in the classical complement pathway). The CVF convertase formed is not susceptible to regulation by inhibitory factor H or factor I, which results in serum decomplementation. Following decomplementation, the only mechanism of protection against bacteremia by antibody should be dependent on Fc-mediated opsonophagocytosis (OPA). The appropriate sublethal decomplementing dose of CVF was determined by injecting rat pups with various doses of CVF i.p. and testing complement activity in serum samples taken from the rats 24 h after administration of the injection. Results (data not shown) from a total complement activity assay (23) determined that 10 μg of CVF is a safe and sufficient dose with which to reduce the complement activity in rat serum. As a control, heat-inactivated (56°C for 30 min) CVF was used.
Challenge of CVF-treated neonate rats.
Four groups of 10 3- to 4-day-old Sprague-Dawley rats were used in the neonate rat challenge model. Two groups were treated with a sublethal dose of 10 μg of CVF 1 h prior to passive immunization with either human adult serum 3 or 16 (from Table 2). Two other groups of rats only received either serum 3 (negative control) or serum 16 (positive control). Twenty hours after the administration of CVF, all rats were challenged with a dose of approximately 5.0 log10 CFU of a rat-passaged strain of N. meningitidis serogroup C strain C11. Three hours after the challenge, rats were sacrificed and bled by cardiac puncture and blood was diluted, plated on GCK agar plates, and incubated at 36°C with 5% CO2. The level of bacteremia in rats was determined by counting colonies on plates inoculated with rat blood.
RESULTS
Anti-MnCPS IgG concentration and serum bactericidal activity.
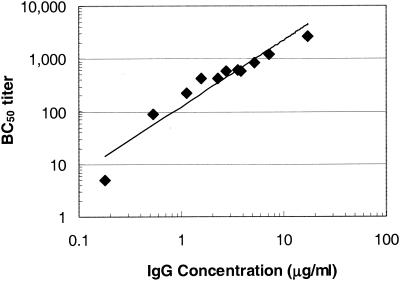
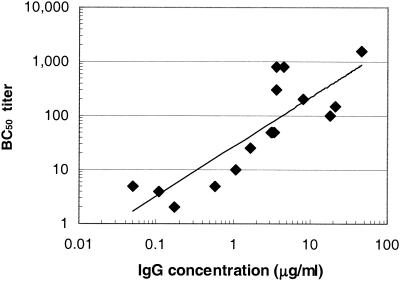
To examine the relationship between the anti-MnCPS-specific IgG concentration and serum bactericidal activity in the neonate rat challenge model, serum pools were created with samples collected from human infants previously immunized with three doses of an MnCC vaccine (Meningitec) (Table 1) or human adults who received a single dose of quadrivalent MnCPS vaccine (Table 2). Serum samples were assayed for total IgG specific for MnCPS and for the capacity to mediate bactericidal activity in vitro. As shown in Table 1, the concentration of MnCPS-specific IgG in the infant serum samples ranged from 0.18 to 17.31 μg/ml while the serum bactericidal activity, reported as the bactericidal titer, ranged from <10 to 2,695 for these serum pools. As depicted in Fig. 1, the data obtained from the infant serum samples indicate a direct relationship between the anti-MnCPS IgG concentration and the bactericidal titer (r = 0.9960). This observation is consistent with the analysis of individual infant serum samples previously reported (46). As shown in Table 2, the concentrations of anti-MnCPS-specific IgG in all of the serum samples from adults ranged from 0.05 (undetectable) to 45.5 μg/ml and the corresponding bactericidal titers ranged from <4 to 1,546. The correlation between the anti-MnCPS IgG concentration and the bactericidal titer of the adult serum samples was reduced (r = 0.708) (Fig. 2) compared to the correlation observed for infant samples.
FIG. 1.
Linear relationship between the anti-MnCPS IgG concentration and the bactericidal titer of 11 pools of human infant serum samples (r = 0.9960). Any bactericidal titer of <10 was assigned a value of 5 (one half of the lowest serum dilution tested). BC50 titer, reciprocal of the highest dilution of antiserum that kills ≥50% of the target cells introduced into the SBA.
FIG. 2.
Correlation between the anti-MnCPS IgG concentration and the bactericidal titer of 16 different adult serum samples (r = 0.708). Any bactericidal titer of <4 was assigned a value of 2 (one half of the lowest serum dilution tested). BC50 titer, reciprocal of the highest dilution of antiserum that kills ≥50% of the target cells introduced into the SBA.
Protection in the challenge model.
The neonate rat challenge model was used to assess the protective capacity of sera from human infants immunized with an MnCC vaccine by using test conditions optimized with serum samples from adults who had received a quadrivalent MnCPS vaccine. As shown in Tables 1 and 2, serum pools or individual serum samples were passively transferred into groups of neonate rats that were subsequently challenged with a bacteremia-inducing dose of an N. meningitidis serogroup C inoculum. The quantity of antibody administered to rat pups was 0.1 ml of a 1:5 dilution of the original serum sample, except for a negative control serum pool that was received in a 1:20 dilution (final dilutions in rat serum, 1:50 and 1:200). The reductions in bacteremia observed for the human infant and individual adult serum panel ranged from 0.52 to 3.72 log10 and from 0.37 to 4.07 log10, respectively. The relationship between the IgG concentration or SBA activity and the reduction in bacteremia was examined. Interestingly, seventy-five (94.9%) of 79 neonate rats (individual animal data not shown) that passively received ≥0.031 μg of IgG in infant serum had a ≥2-log10 reduction in bacteremia, whereas of the animals that received <0.031 μg of IgG, only 4 (10.3%) of 39 rats showed a similar reduction in bacteremia. Similarly, 63 (92.6%) of 68 rat pups that received ≥0.072 μg of N. meningitidis serogroup C-specific IgG in adult serum samples (regardless of whether or not the adult had received MnCPS vaccine; Table 2) had a ≥2-log10 reduction in bacteremia, whereas of the animals that received <0.072 μg of anti-MnCPS IgG in adult serum, only 5 (5.6%) of 90 rats showed a similar reduction in bacteremia. A similar threshold of protection was observed when the estimated bactericidal titer of rat serum after passive transfer (Tables 1 and 2) was considered. The bactericidal titers of rat pup serum samples were estimated by multiplying the original serum sample's bactericidal titer by the final serum dilution factor. Rat pups that received infant serum and had a calculated bactericidal titer of ≥8.5 had a ≥2-log10 reduction in bacteremia, and rats that had a calculated bactericidal titer of <8.5 did not show a ≥2-log10 reduction in bacteremia (Table 1). Similarly, rat pups that received adult serum and had a calculated bactericidal titer of ≥12.8 had a ≥2-log10 reduction in bacteremia and rats that had a calculated bactericidal titer of <12.8 did not show a ≥2-log10 reduction in bacteremia.
Because of the variable bactericidal titers obtained with the adult serum samples relative to the IgG concentrations measured by the ELISA (Fig. 2), it is difficult to assess potential differences in the protective levels of adult serum compared to those of serum samples from infants immunized with MnCC vaccine. However, the anti-MnCPS IgG determinations, which are more precise, do suggest that a lower concentration of antibodies in infants postimmunization with conjugate vaccine are effective in reducing bacteremia, compared to IgG from adults postimmunization with MnCPS. Irrespective of the serologic method used, passive protection in the infant rat challenge model was observed to be dependent on the quantity and, presumably, quality (concentration and function) of anti-MnCPS IgG antibody administered.
The important role of complement factor C3 in protection against bacteremia.
In this study, two groups of 10 rat pups were treated with CVF and subsequently administered either a protective dose of adult normal human serum or immune serum (sera 3 [negative control] and 16 [positive control], respectively, in Table 2). In parallel, two other groups that were not treated with CVF were injected with either the negative or the positive control serum. Eighteen hours later, all groups were challenged with a bacteremia-inducing dose of a rat-passaged strain of N. meningitidis serogroup C strain C11. Results of this study demonstrate the importance of the classical complement pathway in protection against bacteremia. As depicted in Table 3, only the group of rats that received immune human serum 16 (positive control) had a ≥2-log10 reduction in bacteremia. Animals that received a protective dose of human serum 16 and were treated with CVF had levels of bacteremia equivalent to those of the negative control group (serum 3).
TABLE 3.
Complement depletion in neonate rats with CVF affects antibody-dependent serum bactericidal activity and protection in the neonate rat model of bacteremia
| Serum samplea | Adult serum in rats
|
Reduction in bacteremia (log10)d
|
||
|---|---|---|---|---|
| Total anti-MnCPS IgG (μg/rat)b | Bactericidal titer (calculated)c | Normal rat pups | CVF-treated rat pups | |
| 3 | 0.003 | <0.08 | 0.00e | 0.83 |
| 16 | 0.91 | 30.72 | 4.07f | 0.42 |
Individual serum samples used for passive transfer experiments were from healthy nonimmunized adults (serum 3 in Table 2) and serum from individuals that received one dose of a tetravalent meningococcal PS vaccine (A/C/Y/W-135) (serum 16 in Table 2).
Amount of anti-MnCPS-specific IgG passively transferred into rat pups (1:50 dilution of the IgG concentration of individual serum) 18 to 20 h prior to a challenge with group C N. meningitidis strain C11.
Calculated bactericidal titer of neonate rat serum after passive transfer (bactericidal titer of human adult sera ÷ serum dilution factor of 50).
Values represent the average log10 reduction in bacteremia in normal neonate rats or rat pups treated with CVF.
Negative control group.
Significantly different (P < 0.05 as determined by the Student t test) from values obtained from a group of normal rat pups (not treated with CVF).
DISCUSSION
Recently, Ramsey et al. described the efficacy in teenagers and toddlers of a licensed MnCC vaccine introduced in the United Kingdom at the end of 1999 (36). The observed efficacy of the MnCC vaccine is most probably a result of many host immune responses, including complement-mediated, antibody-dependent bactericidal activity. According to Borrow et al. (3), efficacy can be assumed if individuals exhibit a fourfold rise in bactericidal titer after vaccination or have a bactericidal titer of ≥8 in assays in which rabbit serum is the source of the complement used. There is no question that effective MnCC vaccines elicit antibodies that fix complement that results in bacteriolysis or OPA, a fact that is easily demonstrated in in vitro assays. In the study reported here we have described how results of a relevant in vitro assay translate into protection in neonate rats in a challenge model of bacteremia and also demonstrated the importance of complement-mediated antibody-dependent bactericidal activity. In this study, a direct correlation was found between the IgG concentration and bactericidal titer for pools of infant serum (r =0.9960; Fig. 1). The sample size (n = 11 pools) was limited, but the correlation was not unexpected. We previously tested serum samples from 45 individual infants and reported a good correlation between the results obtained in an ELISA and the results of an SBA (46). The MnCC vaccine elicits immune responses much greater than the protective thresholds previously established (17), and thus, serum samples from age-matched nonimmunized infants enabled us to demonstrate a linear relationship between the two assay methods. This finding would not be surprising if one considered complement-fixing antibodies a subset of the total anti-MnCPS-specific IgG. Our observed correlation between the IgG concentration and the bactericidal titer does not dispute the observations made by others where strong correlations were not found (21). In fact, in this study, a relatively poor correlation (r = 0.708) was found between the bactericidal titer and the level of anti-MnCPS-specific IgG antibody in adult serum samples. A number of factors may contribute this observation: bactericidal activity can occur because of other antibodies or factors in addition to IgG antibodies specific to the PS that is the outcome of the ELISA measurement, the ELISA may detect low-avidity antibodies or antibodies that are not associated with functional activity as measured in the SBA, and additionally, the number of adult serum samples tested in this study was small (n = 16).
Experimental infection of neonate rats with pathogenic Haemophilus influenzae type b has been previously described as part of the effort to evaluate the protective potential of infant conjugate vaccine-elicited polyclonal antibodies to H. influenzae PS (20, 29). To date, a study of the protective activity of human infant antiserum specific for MnCPS has not been described. When challenged with N. meningitidis serogroup C bacteria, neonate rats usually become bacteremic and may even succumb to meningitis and/or death, depending on the design of the model (40). In our laboratory, bacteremia could be achieved reproducibly 3 h after i.p. infection of neonate rats with a challenge dose of ∼5.0 log10 CFU. Similar to other investigators (40), we found it necessary to passage challenge strain C11 one time in neonate rats in order to get a uniformly high level (∼4.5 log10 CFU/ml of blood) of bacteremia in unprotected rats. In the neonatal rat model of bacteremia with N. meningitidis serogroup C, passive immunization with human infant or adult serum samples with sufficient quantities of anti-MnCPS IgG was protective. Passive immunization of rats with infant serum having anti-MnCPS IgG concentrations of ≥0.031 μg/ml and a corresponding bactericidal titer (calculated) of ≥1:8.5 had a statistically significant reduction bacteremia after a challenge. Bactericidal titers of <1:8.5 (calculated) could not reduce the level of bacteremia in passively immunized rat pups after a challenge (Table 1). Similarly, dose-dependent protection from bacteremia was also observed after passive transfer of adult serum. In this limited study, it was determined that rats that received ≥0.072 μg of adult MnCPS-specific IgG had reduced bacteremia levels of ≥2.0 log10 CFU/ml of blood, compared to animals that received serum with undetectable levels of specific IgG (Table 2).
While it would have been interesting to compare the results obtained in this study with those obtained with immune serum samples from infants who received an MnCPS vaccine, such samples are not available since the PS vaccine is poorly immunogenic in this age group. However, adult anti-MnCPS immune serum samples were available and were tested in the ELISA, the SBA, and the neonate rat model. As depicted in Fig. 2, adult anti-MnCPS antiserum displayed a poorer correlation between the IgG concentration and the bactericidal titer (r = 0.708) than that obtained with infant serum (Fig. 1), regardless of whether or not the adults had received an MnCPS vaccine. The variability of the adult serum samples (adult age, immunological experience, etc.) makes it difficult to draw conclusions about the comparative functionality of the human infant and adult serum samples used in these studies. One can speculate, though, that the anti-MnCPS IgG antibodies elicited by the conjugate vaccine in infants are more effective at protection in the neonate rat model than is the population of IgG antibodies elicited in adults by the PS vaccine. This hypothesis is supported by the ELISA data in Tables 1 and 2. The ELISA is a more precise test method than the SBA for such purposes because each specimen is quantitated against a standard reference preparation (micrograms per milliliter), while the SBA has incremental titer readouts without normalization to any standard. Other factors contributing to the differences in performance between these two methods have already been discussed above.
To determine if complement is a prerequisite for protection in this bacteremia model, neonate rats were treated with a dose of CVF sufficient to deplete rat serum of terminal complement components (TCC). CVF, at a dose 10 μg per rat, was adequate to consume serum complement, resulting in undetectable levels of complement activity in a total complement activity assay (23). In this study, a direct association was made between passively transferred anti-MnCPS bactericidal antibody and an intact complement system. However, it cannot be assumed that SBA activity is the only mechanism of protection in this model; opsonophagocytosis (OPA) has also been presumed to play a role in protection against N. meningitidis serogroup C in vivo. OPA activity is dependent on C3 and antibody for efficient killing of N. meningitidis serogroup C. Evidence that OPA plays a role in protection has been demonstrated in studies of individuals with TCC deficiency in which MnCPS vaccines have been proven somewhat effective. Protection offered by MnCPS vaccines administered to TCC-deficient individuals has been observed to be dependent on the concentration of antibody specific for MnCPS (2, 31, 44). If it had been available, it would have been interesting to test infant immune serum in neonate rats deficient in any complement factor from C5 through C9 to accurately evaluate the importance of OPA in the neonate rat model.
Studies as early as those of Silverthorne (47) and Goldschneider et al. (17) and as recent as that of Borrow et al. (3) argue that the bactericidal titer can serve as a surrogate marker of protection against invasive disease. This study demonstrates that results obtained from in vitro assays such as the SBA can translate to protection in in vivo animal models. There is a good rationale for supplementing in vitro data with in vivo data. Antibody testing in vivo most closely represents conditions that exist in the disease state. Limitations on serum factors are not as restrictive as those of in vitro assays, and the interactions between the host and pathogen in the model is not unrealistic. However, rat complement and other systemic immune components are not truly representative of humans. The neonate rat model is a sensitive model that can provide support to results obtained by ELISA and the SBA.
Editor: A. D. O'Brien
REFERENCES
- 1.Anderson, E. L., T. Bowers, C. M. Mink, D. J. Kennedy, R. B. Belshe, H. Harakeh, L. Pais, P. Holder, and G. M. Carlone. 1994. Safety and immunogenicity of meningococcal A and C polysaccharide conjugate vaccine in adults. Infect. Immun. 62:3391-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreoni, J., H. Kayhty, and P. Densen. 1993. Vaccination and the role of capsular polysaccharide antibody in prevention of recurrent meningococcal disease in late complement component-deficient individuals. J. Infect. Dis. 168:227-231. [DOI] [PubMed] [Google Scholar]
- 3.Borrow, R., N. Andrews, D. Goldblatt, and E. Miller. 2001. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect. Immun. 69:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow, R., A. J. Fox, K. Cartwright, N. T. Begg, and D. M. Jones. 1999. Salivary antibodies following parenteral immunization of infants with a meningococcal serogroup A and C conjugated vaccine. Epidemiol. Infect. 123:201-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, R., A. J. Fox, P. C. Richmond, S. Clark, F. Sadler, J. Findlow, R. Morris, N. T. Begg, and K. A. Cartwright. 2000. Induction of immunological memory in UK infants by a meningococcal A/C conjugate vaccine. Epidemiol. Infect. 124:427-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramley, J. C., T. Hall, A. Finn, R. B. Buttery, D. Elliman, S. Lockhart, R. Borrow, and I. G. Jones. 2001. Safety and immunogenicity of three lots of meningococcal serogroup C conjugate vaccine administered at 2, 3 and 4 months of age. Vaccine 19:2924-2931. [DOI] [PubMed] [Google Scholar]
- 7.Campagne, G., A. Garba, P. Fabre, A. Schuchat, R. Ryall, D. Boulanger, M. Bybel, G. Carlone, P. Briantais, B. Ivanoff, B. Xerri, and J. P. Chippaux. 2000. Safety and immunogenicity of three doses of a Neisseria meningitidis A + C diphtheria conjugate vaccine in infants from Niger. Pediatr. Infect. Dis. J. 19:144-150. [DOI] [PubMed] [Google Scholar]
- 8.Choo, S., J. Zuckerman, C. Goilav, E. Hatzmann, J. Everard, and A. Finn. 2000. Immunogenicity and reactogenicity of a group C meningococcal conjugate vaccine compared with a group A + C meningococcal polysaccharide vaccine in adolescents in a randomised observer blind controlled trial. Vaccine 18:2686-2692. [DOI] [PubMed] [Google Scholar]
- 9.Costantino, P., S. Viti, A. Podda, M. A. Velmonte, L. Nencioni, and R. Rappuoli. 1992. Development and phase 1 clinical testing of a conjugate vaccine against meningococcus A and C. Vaccine 10:691-698. [DOI] [PubMed] [Google Scholar]
- 10.Craven, D. E., and C. E. Frasch. 1979. Protection against group B meningococcal disease: evaluation of serotype 2 protein vaccines in a mouse bacteremia model. Infect. Immun. 26:110-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.English, M., J. M. MacLennan, J. M. Bowen-Morris, J. Deeks, M. Boardman, K. Brown, S. Smith, J. Buttery, J. Clarke, S. Quataert, S. Lockhart, and E. R. Moxon. 2000. A randomised, double-blind, controlled trial of the immunogenicity and tolerability of a meningococcal group C conjugate vaccine in young British infants. Vaccine 19:1232-1238. [DOI] [PubMed] [Google Scholar]
- 12.Fairley, C. K., N. Begg, R. Borrow, A. J. Fox, D. M. Jones, and K. Cartwright. 1996. Conjugate meningococcal serogroup A and C vaccine: reactogenicity and immunogenicity in United Kingdom infants. J. Infect. Dis. 174:1360-1363. [DOI] [PubMed] [Google Scholar]
- 13.Figueroa, J. E., and P. Densen. 1991. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 4:359-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frantz, I. D. 1942. Growth requirements of the meningococcus. J. Bacteriol. 43:757-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frasch, C. E., L. Parkes, R. M. McNelis, and E. C. Gotschlich. 1976. Protection against group B meningococcal disease. I. comparison of group-specific and type-specific protection in the chick embryo model. J. Exp. Med. 144:319-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frasch, C. E., and J. B. Robbins. 1978. Protection against group B meningococcal disease. III. Immunogenicity of serotype 2 vaccines and specificity of protection in a guinea pig model. J. Exp. Med. 147:629-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez, S., and M. Leinonen. 1991. Neisseria 1990, p. 225-228. In M. Achtman, P. Kohl, C. Marchal, G. Morelli, A. Seiler, and B. Thiesen (ed.), Proceedings of the 7th International Pathogenic Neisseria Conference. Walter de Gruyter & Co., Berlin, Germany.
- 19.Gotschlich, E. C., T. Y. Liu, and M. S. Artenstein. 1969. Human immunity to the meningococcus. III. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J. Exp. Med. 129:1349-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granoff, D. M., E. G. Boies, and R. S. Munson. 1984. Immunogenicity of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine in adults. J. Pediatr. 105:22-27. [DOI] [PubMed] [Google Scholar]
- 21.Granoff, D. M., S. E. Maslanka, G. M. Carlone, B. D. Plikaytis, G. F. Santos, A. Mokatrin, and H. V. Raff. 1998. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin. Diagn. Lab. Immunol. 5:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Infante, J. F., O. Marrero, S. Sifontes, G. Sierra, C. Campa, E. Caro, M. Gutierrez, A. Malberti, V. Capo, M. Farinas, and E. Munoz. 1994. Evaluation of the efficacy of human antimeningococcal immunoglobulin G in infant rats experimentally infected with Neisseria meningitidis group B. Arch. Med. Res. 25:455-461. [PubMed] [Google Scholar]
- 23.Kabat, E. A., and M. M. Mayer. 1961. Experimental immunochemistry, 2nd ed. Charles C Thomas, Springfield, Ill.
- 24.Kellogg, D. S., W. L. Peacock, and W. E. Deacon. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leach, A., P. A. Twumasi, S. Kumah, W. S. Banya, S. Jaffar, B. D. Forrest, D. M. Granoff, D. E. LiButti, G. M. Carlone, L. B. Pais, C. V. Broome, and B. M. Greenwood. 1997. Induction of immunologic memory in Gambian children by vaccination in infancy with a group A plus group C meningococcal polysaccharide-protein conjugate vaccine. J. Infect. Dis. 175:200-204. [DOI] [PubMed] [Google Scholar]
- 26.Lieberman, J. M., S. S. Chiu, V. K. Wong, S. Partridge, S.-J. Chang, C.-Y. Chiu, L. L. Gheesling, G. M. Carlone, and J. I. Ward. 1996. Safety and immunogenicity of a serogroups A/C Neisseria meningitidis oligosaccharide-protein conjugate vaccine in young children. JAMA 275:1499-1503. [PubMed] [Google Scholar]
- 27.MacLennan, J., S. Obaro, J. Deeks, D. Lake, C. Elie, G. Carlone, E. R. Moxon, and B. Greenwood. 2001. Immunologic memory 5 years after meningococcal A/C conjugate vaccination in infancy. J. Infect. Dis. 183:97-104. [DOI] [PubMed] [Google Scholar]
- 28.MacLennan, J., S. Obaro, J. Deeks, D. Williams, L. Pais, G. Carlone, R. Moxon, and B. Greenwood. 1999. Immune response to revaccination with meningococcal A and C polysaccharides in Gambian children following repeated immunisation during early childhood. JAMA 17:3086-3093. [DOI] [PubMed] [Google Scholar]
- 29.Madore, D. V., C. L. Johnson, D. C. Phipps, M. G. Myers, R. Eby, and D. H. Smith. 1990. Safety and immunogenicity of Haemophilus influenzae type b oligosaccharide-CRM197 conjugate vaccine in infants aged 15 to 23 months. Pediatrics 86:527-534. [PubMed] [Google Scholar]
- 30.Matthews, N., J. M. Stark, P. S. Harper, J. Doran, and D. M. Jones. 1980. Recurrent meningococcal infections associated with a functional deficiency of the C8 component of human complement. Clin. Exp. Immunol. 39:53-59. [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan, B. P., and A. Orren. 1998. Vaccination against meningococcus in complement-deficient individuals. Clin. Exp. Immunol. 114:327-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mountzouros, K. T., and A. P. Howell. 2000. Detection of complement-mediated antibody-dependent bactericidal activity in a fluorescence-based serum bactericidal assay for group b Neisseria meningitidis. J. Clin. Microbiol. 38:2878-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nassif, X., J. C. Mathison, E. Wolfson, J. A. Koziol, R. J. Ulevitch, and M. So. 1992. Tumour necrosis factor alpha antibody protects against lethal meningococcaemia. Mol. Microbiol. 6:591-597. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson, A., and I. H. Lepoe. 1979. Host defense against Neisseria meningitidis requires a complement-dependent bactericidal activity. Science 205:298-299. [DOI] [PubMed] [Google Scholar]
- 35.Petersen, B. H., T. J. Lee, R. Snyderman, and G. F. Brooks. 1979. Neisseria meningitidis and Neisseria gonorrhoeae bacteremia associated with C6, C7, or C8 deficiency. Ann. Intern. Med. 90:917-920. [DOI] [PubMed] [Google Scholar]
- 36.Ramsay, M. E., N. Andrews, E. B. Kaczmarski, and E. Miller. 2001. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 357:195-196. [DOI] [PubMed] [Google Scholar]
- 37.Rennels, M. B., K. M. Edwards, H. L. Keyserling, K. Reisinger, M. M. Blatter, S. A. Quataert, D. V. Madore, I. Chang, F. J. Malinoski, J. G. Hackell, and P. R. Paradiso. 2001. Safety and immunogenicity of four doses of Neisseria meningitidis group C vaccine conjugated to CRM197 in United States infants. Pediatr. Infect. Dis. J 20:153-159. [DOI] [PubMed] [Google Scholar]
- 38.Richmond, P., D. Goldblatt, P. C. Fusco, J. D. Fusco, I. Heron, S. Clark, R. Borrow, and F. Michon. 1999. Safety and immunogenicity of a new Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine in healthy adults. Vaccine 18:641-646. [DOI] [PubMed] [Google Scholar]
- 39.Ross, S. C., and P. Densen. 1984. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine 63:243-273. [PubMed] [Google Scholar]
- 40.Saukkonen, K. 1988. Experimental meningococcal meningitis in the infant rat. Microb. Pathog. 4:203-211. [DOI] [PubMed] [Google Scholar]
- 41.Saukkonen, K., H. Abdillahi, J. T. Poolman, and M. Leinonen. 1987. Protective efficacy of monoclonal antibodies to class 1 and class 3 outer membrane proteins of Neisseria meningitidis B:15:P1.16 in infant rat infection model: new prospects for vaccine development. Microb. Pathog. 3:261-267. [DOI] [PubMed] [Google Scholar]
- 42.Saukkonen, K., M. Leinonen, H. Abdillahi, and J. T. Poolman. 1989. Comparative evaluation of potential components for group B meningococcal vaccine by passive protection in the infant rat and in vitro bactericidal assay. Vaccine 7:325-328. [DOI] [PubMed] [Google Scholar]
- 43.Saukkonen, K., M. Leinonen, H. Kayhty, H. Abdillahi, and J. T. Poolman. 1988. Monoclonal antibodies to the rough lipopolysaccharide of Neisseria meningitidis protect infant rats from meningococcal infection. J. Infect. Dis. 158:209-212. [DOI] [PubMed] [Google Scholar]
- 44.Schlesinger, M., R. Greenberg, J. Levy, H. Kayhty, and R. Levy. 1994. Killing of meningococci by neutrophils: effect of vaccination on patients with complement deficiency. J. Infect. Dis. 170:449-453. [DOI] [PubMed] [Google Scholar]
- 45.Sifontes, S., J. F. Infante, P. Perez, E. Caro, G. Sierra, and C. Campa. 1997. The hyperferremic mouse model of the evaluation of the effectiveness of VA-MENGOC-BC against Neisseria meningitidis B clinical isolates. Arch. Med. Res. 28:41-45. [PubMed] [Google Scholar]
- 46.Sikkema, D. J., K. E. Friedman, B. Corsaro, A. Kimura, S. W. Hildreth, D. V. Madore, and S. A. Quataert. 2000. Relationship between serum bactericidal activity and serogroup-specific IgG concentration for adults, toddlers and infants immunized with Neisseria meningitidis serogroup C vaccines. Clin. Diagn. Lab. Immunol. 7:764-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silverthorne, N. 1937. The bactericidal power of blood and protection against the meningococcal infection. J. Immunol. 33:51-56. [Google Scholar]
- 48.Thomas, L., and J. H. Dingle. 1943. Investigations of meningococcal infection. III. The bactericidal action of normal and immune serums for the meningococcus. J. Clin. Investig. 22:375-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Twumasi, P. A., Jr., S. Kumah, A. Leach, T. J. D. O'Dempsey, S. J. Ceesay, J. Todd, C. V. Broome, G. M. Carlone, L. B. Pais, P. K. Holder, B. D. Plikaytis, and B. M. Greenwood. 1995. A trial of a group A plus group C meningococcal polysaccharide-protein conjugate vaccine in African infants. J. Infect. Dis. 171:632-638. [DOI] [PubMed] [Google Scholar]