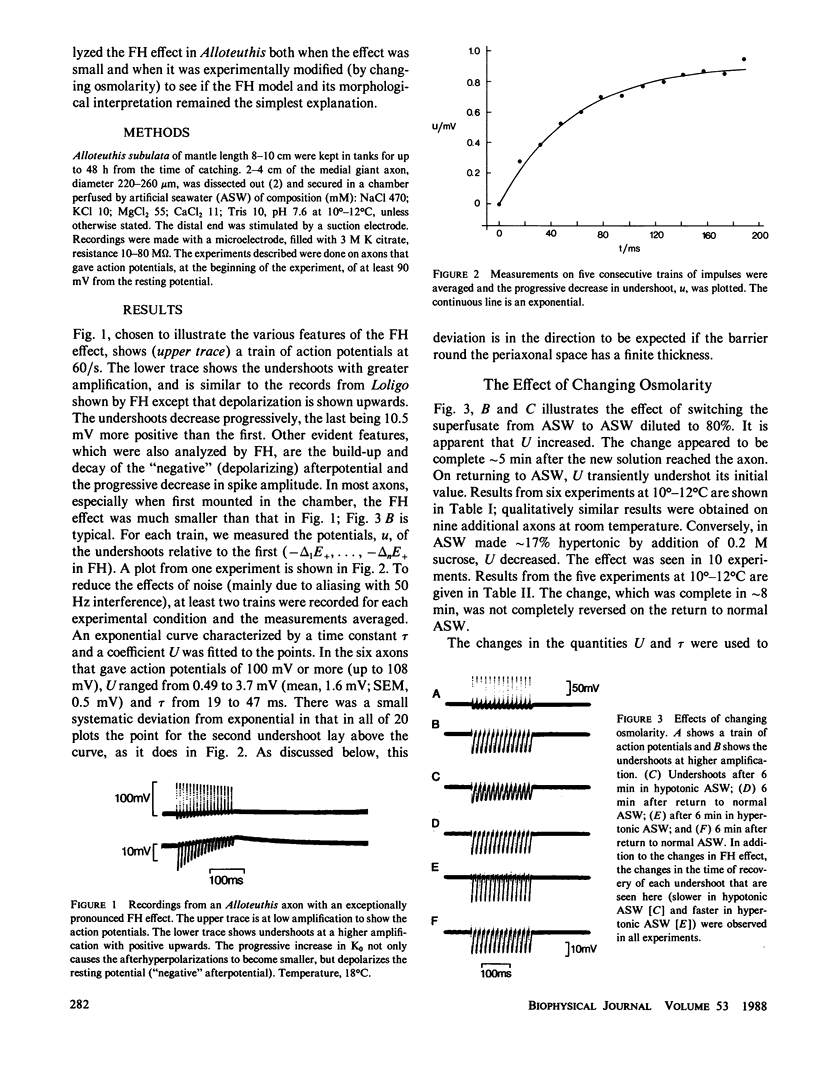
Abstract
In a train of impulses in squid giant axon, accumulation of extracellular potassium causes successive afterhyperpolarizations to be progressively less negative. In Loligo, Frankenhaeuser and Hodgkin had satisfactorily accounted for the characteristics of this effect with a model in which the axon is surrounded by a space, width theta, and a barrier of permeability P. In axons isolated from Alloteuthis, we found that the model fitted the observations quite well. Superfusing the axon with hypotonic artificial seawater (ASW) caused theta and P to decrease, and, conversely, hypertonic ASW caused them to increase: this would be the case if both the space and the pathway through the barrier were extracellular. In some cases, in normal ASW, the afterhyperpolarizations in a train decreased very little, less than 0.7 mV. In these extreme cases, theta was estimated to be 190 nm and P to be 7 x 10(-4) cm s-1, both several times the values of 30 nm and 6 x 10(-5) cm s-1 estimated by Frankenhaeuser and Hodgkin. We suggest that in vivo the periaxonal space may be considerably wider than that seen in conventionally fixed squid tissue.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott N. J., Lieberman E. M., Pichon Y., Hassan S., Larmet Y. Periaxonal K+ regulation in the small squid Alloteuthis. Studies on isolated and in situ axons. Biophys J. 1988 Feb;53(2):275–279. doi: 10.1016/S0006-3495(88)83089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman W. J., Jr, Moses J., Rive R. V. An anatomical basis for the resistance and capacitance in series with excitable membrane of the squid giant axon. J Neurocytol. 1977 Dec;6(6):621–646. doi: 10.1007/BF01176377. [DOI] [PubMed] [Google Scholar]
- Adelman W. J., Jr, Palti Y., Senft J. P. Potassium ion accumulation in a periaxonal space and its effect on the measurement of membrane potassium ion conductance. J Membr Biol. 1973 Nov 8;13(4):387–410. doi: 10.1007/BF01868237. [DOI] [PubMed] [Google Scholar]
- Binstock L., Goldman L. Rectification in instantaneous potassium current-voltage relations in Myxicola giant axons. J Physiol. 1971 Sep;217(3):517–531. doi: 10.1113/jphysiol.1971.sp009583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J. A., Tsacopoulos M. Potassium activity in photoreceptors, glial cells and extracellular space in the drone retina: changes during photostimulation. J Physiol. 1979 May;290(2):525–549. doi: 10.1113/jphysiol.1979.sp012788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg B. Preservation of extracellular space during fixation of the brain for electron microscopy. Tissue Cell. 1980;12(1):63–72. doi: 10.1016/0040-8166(80)90052-x. [DOI] [PubMed] [Google Scholar]
- Evans P. D., Reale V., Villegas J. The role of cyclic nucleotides in modulation of the membrane potential of the Schwann cell of squid giant nerve fibre. J Physiol. 1985 Jun;363:151–167. doi: 10.1113/jphysiol.1985.sp015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Medwin A. R. A study of the mechanisms by which potassium moves through brain tissue in the rat. J Physiol. 1983 Feb;335:353–374. doi: 10.1113/jphysiol.1983.sp014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W. Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol Sci. 1967 Jun 6;168(1010):1–21. doi: 10.1098/rspb.1967.0047. [DOI] [PubMed] [Google Scholar]
- Kukita F., Yamagishi S. Effects of an outward water flow on potassium currents in a squid giant axon. J Membr Biol. 1983;75(1):33–44. doi: 10.1007/BF01870797. [DOI] [PubMed] [Google Scholar]
- Landowne D., Scruggs V. The temperature dependence of the movement of potassium and chloride ions associated with nerve impulses. J Physiol. 1976 Jul;259(1):145–158. doi: 10.1113/jphysiol.1976.sp011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem R. D., Hammerschlag R., Brancho H., Orkand R. K. Influence of potassium ions on accumulation and metabolism of (14C)glucose by glial cells. Brain Res. 1975 Mar 28;86(3):499–503. doi: 10.1016/0006-8993(75)90903-8. [DOI] [PubMed] [Google Scholar]
- Shrager P., Starkus J. C., Lo M. V., Peracchia C. The periaxonal space of crayfish giant axons. J Gen Physiol. 1983 Aug;82(2):221–244. doi: 10.1085/jgp.82.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimers J. R., Bezanilla F., Taylor R. E. Sodium channel gating currents. Origin of the rising phase. J Gen Physiol. 1987 Apr;89(4):521–540. doi: 10.1085/jgp.89.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLEGAS R., VILLEGAS G. M. Characterization of the membranes in the giant nerve fiber of the squid. J Gen Physiol. 1960 May;43:73–103. doi: 10.1085/jgp.43.5.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harreveld A., Steiner J. Extracellular space in frozen and ethanol substituted central nervous tissue. Anat Rec. 1970 Jan;166(1):117–129. doi: 10.1002/ar.1091660109. [DOI] [PubMed] [Google Scholar]


