Abstract
The resolution of Chlamydophila abortus (Chlamydia psittaci serotype 1) infection is dependent on gamma interferon and CD8+ T cells, and classically, B cells have been considered to play a minimal role in host defense. The role of B cells in the immune response was studied by using a model of infection in mice with genetically modified immunoglobulin M transmembrane domains (μMT). In the absence of B cells, infection with C. abortus leads to an acute severe fatal disease that involves a disseminated intravascular coagulation syndrome. μMT mice displayed an increased level of proinflammatory cytokines in serum, and an increased number of neutrophils was observed in the lesions. The possible deleterious role of neutrophils in the pathogenesis of disease in μMT mice was determined by depletion of the neutrophils with the monoclonal antibody RB6-8C5. This led to an enhancement of the bacterial burden and early mortality in both μMT and wild-type mice, while necrotic lesions remained. Analysis of the presence of immunoregulatory cytokines showed significantly lower levels of transforming growth factor β in the sera of μMT mice. However, mice lacking mature B cells were able to establish a specific immune response that protected them from a secondary challenge. Taken together, these data suggest an immunomodulatory role for B cells in the early events of C. abortus primary infection that can protect mice against an exaggerated inflammatory response.
Chlamydophila abortus (Chlamydia psittaci serotype 1) is an obligate intracellular bacterium and the etiological agent of enzootic abortion in small ruminants. The disease is serious because of the economic losses it causes and the potential zoonotic risk for pregnant women (36). Mouse models have been widely used to study the immune response to this bacterium (5, 7, 10, 30) and the pathogenesis of chlamydial abortion. It has been observed that the inoculation of pregnant mice with C. abortus causes late-term abortions similar to those observed in cases of natural or experimentally induced abortion in ruminants (6).
In experimental infection, the establishment of an effective immune response after the systemic spread of C. abortus is able to eliminate the infection in every organ except the placenta of pregnant animals, where the multiplication of the bacterium induces abortion (6). The effective immune response observed in the spleen and liver involves neutrophils, which act as a first line of defense (3) and participate in the recruitment of other leukocyte subpopulations (32). Also, a strong Th1-like specific immune response is rapidly induced, involving the production of proinflammatory cytokines, especially gamma interferon (IFN-γ) (30), and activation of T cells, with CD8+ T cells playing an important role in the resolution of the infection (7, 10). However, it has been reported recently that an interleukin 12 (IL-12)-dependent overproduction of IFN-γ may result in an increase in morbidity and pathology (9).
The role of B cells in the immune response against C. abortus has been poorly studied, although it has been demonstrated that the passive transfer of specific antibodies protects pregnant animals against chlamydial abortion (11). Thus, B cells not only play a pivotal role in the production and amplification of humoral immune responses but also act as antigen-presenting cells (APCs) in the generation of T-cell-mediated immune responses (22). With the development of B-cell-deficient mice (μMT) through disruption of the transmembrane portion of the μ chain gene (21), the functions of B cells other than antibody production can readily be studied in infections in which the cellular immune response is important. Recent studies have identified several of these functions in the response against parasites, bacteria, and viruses. B cells play an immunomodulatory role in Schistosoma mansoni infection (19) and are involved in protective immunity against Salmonella enterica serovar Typhimurium (31). The lack of B cells leads to a suboptimal vaccinal response to Toxoplasma gondii (38) and to a delay in dissemination of and development of lung pathology in response to Mycobacterium tuberculosis (1). B cells have a role in early protective immunity against Francisella tularensis infection (8), play a nonantibody role during primary (26) and secondary (23) respiratory infection with Bordetella pertussis, and mediate innate and CD4+-T-cell-specific immune responses in herpes simplex virus infections (12). Several studies have been performed with another species of the family Chlamydiaceae, Chlamydia trachomatis, producing contradictory results (20, 33, 40, 44). Thus, in order to deepen our knowledge of the immune mechanisms involved in C. abortus clearance, it is necessary to study the potential functions of B cells in primary and secondary infection. In this study, we placed special emphasis on the relationship between the lack of B cells and neutrophilia, which has been described in the immune response to some intracellular pathogens (2, 39), and on the incidence of this correlation in the early proinflammatory cytokine response and subsequent inflammation-associated pathology.
MATERIALS AND METHODS
Mice.
Eight-week-old female C57BL/6J (H-2b) mice and μMT mice of the same genotype were purchased from Harlan UK Limited (Blackthorn, United Kingdom) and Jackson Laboratory (Bar Harbor, Maine), respectively. According to the results of routine screening procedures performed by the suppliers, these mice were free of common viral and bacterial pathogens.
Bacteria and infection.
Mice were infected with the abortion-causing C. abortus strain AB7, which had been isolated in a case of ovine abortion (37). The bacteria were propagated in the yolk sacs of developing chick embryos and titrated by counting the number of inclusion-forming units (IFUs) on McCoy cells, as described by Buendía et al. (3). Standardized aliquots were frozen at −80°C until use.
Wild-type (WT) and μMT mice were challenged by intraperitoneal (i.p.) injection with 1 × 106 or 2 × 104 IFUs of C. abortus (see Results) in 0.1 ml of phosphate-buffered saline (PBS), pH 7.2 (0.1 M). For each experiment, one group of mice from each strain was inoculated with 0.1 ml of sterile PBS to serve as the noninfected group. At 2 and 4 days postinfection (p.i.), mice of each strain were killed and samples of different organs and sera were collected under aseptic conditions.
Isolation of C. abortus.
The course of infection was also evaluated by counting the number of IFUs from the liver after isolation of C. abortus on McCoy cell monolayers according to the method previously described by Buendía et al. (3). One lobe of the liver was examined, and the number of IFUs per gram was calculated. The detection limit was 2.6-log IFUs per sample.
Histopathology.
Liver, spleen, lung, and kidney samples were collected and fixed in 4% paraformaldehyde in PBS. After being dehydrated and embedded in paraffin wax at 56°C, sections (5 μm thick) were cut, stained with hematoxylin and eosin, and analyzed for histopathological changes.
Immunohistochemistry.
To visualize chlamydial antigen in paraffin sections, immunohistochemical staining was carried out with an anti-chlamydial-lipopolysaccharide-specific biotinylated mouse monoclonal antibody (MAb), as previously described (6), by using the avidin-biotin-peroxidase complex (ABC) method according to the instructions of the manufacturer (Vector Laboratories, Burlingame, Calif.). A positive reaction was demonstrated by the precipitation of diaminobenzidine tetrahydrochloride. Sections were subsequently stained with hematoxylin.
In vivo neutrophil depletion.
The hybridoma producing the RB6-8C5 MAb (41) was provided by R. L. Coffman (DNAX Research Institute, Palo Alto, Calif.). The rat immunoglobulin G2b (IgG2b) RB6-8C5 MAb was obtained from the ascitic fluid of pristane-primed homozygous nude mice (Harlan) injected with 107 hybridoma cells. The MAb was concentrated by saturated ammonium sulfate precipitation, followed by dialysis against PBS (pH 7.2) and filtration at 0.22 μm. The MAb was purified by chromatography on a protein G column according to the instructions of the manufacturer (Sigma, Madrid, Spain). Protein concentration was estimated by use of a modified Lowry method using the BCA-1 procedure (Sigma).
Neutrophil-depleted mice received 1 mg of RB6-8C5 MAb intravenously 6 h before the challenge and subsequently on days 1 and 3 p.i. Nondepleted mice received rat IgG (Sigma) at the same time, by the same route, and at the same dosage. Neutrophil depletion in the sacrificed mice was assessed by flow cytometry analysis, as described by Montes de Oca et al. (32).
Serum transaminase assay.
To measure the amount of the liver-associated enzyme alanine transaminase (ALT) in serum, a protocol modified from a commercial kit (Sigma) was employed. Briefly, in a 96-well plate, 4 μl of serum was added to 20 μl of dl-alanine (0.2 mol/liter) and α-ketoglutaric acid (1.8 mmol/liter); the plate was shaken and the mixture was incubated for 30 min at 37°C. Then, 20 μl of 2,4-dinitrophenylhydrazine (DNP) was added, and the mixture was incubated for an additional 20 min at room temperature. Finally, the reaction was halted by the addition of 200 μl of 0.4 N NaOH, and sample absorbances were measured at 492 nm after 5 min.
Spleen cell culture.
The caudal halves of the spleens taken from mice were ground through a sieve with a 100-μm mesh, depleted of erythrocytes by ammonium chloride treatment, and washed with RPMI 1640 medium (Gibco, Paisley, United Kingdom). After the cells were counted, 5 × 105 cells/ml were resuspended in RPMI 1640 culture medium supplemented with 10% fetal calf serum (Gibco), 200 mM l-glutamine, 5 × 10−5 M 2-mercaptoethanol, 2.5 μg of amphotericin B (Fungizone)/ml, 100 IU of penicillin/ml, and 10 μg of streptomycin/ml (all from Sigma) and plated in 24-well plates (Corning, Cambridge, Mass.) at 1 ml/well. For specific activation, elementary bodies of C. abortus (Chl-Ag, 50 μg/ml) purified on a Urografin (Schering, Madrid, Spain) gradient as previously described (4) were added. In order to compare the unspecific activation of lymphocytes, 5 μg of concanavalin A/ml (Sigma) was added. Incubation was performed for 48 h at 37°C in a humidified atmosphere of 5% CO2.
Cytokine analysis.
The presence of inflammatory cytokines in sera, splenocyte culture supernatants, and liver tissue homogenates was assessed. To obtain liver homogenates, snap-frozen liver samples were thawed on ice, weighed, and homogenized in a solution containing 2 mg of protease inhibitor (Complete; Roche Diagnostics, Barcelona, Spain) per ml of PBS. Cell-free tissue homogenates obtained after centrifugation were used in the determinations. IFN-γ concentrations were determined using a sandwich enzyme-linked immunosorbent assay (ELISA). The capture antibody used was R4-6A2, and the biotinylated detection antibody was XMG1.2. All antibodies were purchased from PharMingen (San Diego, Calif.). The bound biotinylated antibody was detected with a horseradish peroxidase-streptavidin conjugate (PharMingen) and a soluble substrate, ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid); Sigma]. The optical density at 405 nm was read. The levels of the proinflammatory cytokines, tumor necrosis factor alpha (TNF-α) and IL-6, and immunoregulatory cytokine IL-10 were determined using ELISA kits obtained from Endogen (Woburn, Mass.). To measure the level of TGF-β in serum, samples were acid treated and a commercial ELISA kit obtained from Promega (Madison, Wis.) was used according to the manufacturer's instructions. The cytokine levels in liver homogenates were normalized to the weight of the liver sample.
Secondary infection.
To study the immune response to secondary infection, WT and μMT mice were given a secondary challenge of 106 IFUs 6 weeks after a first challenge with 2 × 104 IFUs. These mice were killed 5 days after the secondary infection challenge, and samples of different organs and sera were removed.
Evaluation of antibody responses to C. abortus antigens.
Sera from reinfected mice were collected and stored at −20°C until examination by ELISA for IgG2a and IgG1 responses as previously described (10).
Statistical analysis.
Evaluation of statistical differences between data was performed by the two-tailed Student t test. A P value of <0.01 was considered significant.
RESULTS
μMT mice display an increased susceptibility to C. abortus infection.
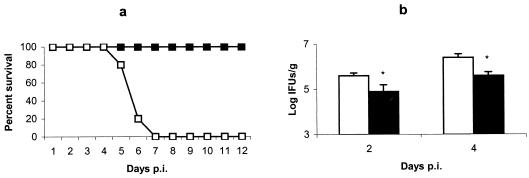
In order to determine the susceptibility of both mouse strains to C. abortus, mice were infected with 106 IFUs and survival was recorded daily (Fig. 1a). μMT mice succumbed to infection within 5 to 7 days p.i. No mortality was observed in WT mice, although there were signs of illness, such as ruffled fur, lethargy, and huddling, from 3 days to 9 or 10 days p.i. The isolation of C. abortus from the liver pointed to a significantly higher level of infection in the μMT mice than in the WT mice at 2 and 4 days p.i. (Fig. 1b).
FIG. 1.
Evolution of C. abortus infection in μMT and WT mice. (a) Survival of μMT (□) and WT (▪) mice after infection. Groups of eight mice were infected with 106 IFUs of C. abortus. Mice were monitored daily, and mortality rates were calculated. The experiment was repeated twice, and the data shown are the results for two groups of mice of each strain (16 mice per strain). (b) Quantitative isolation of C. abortus on homogenized tissues from livers of μMT (white columns) and WT (black columns) mice collected at days 2 and 4 p.i. The number of inclusions was visualized by indirect immunofluorescence. The results are the means and SD for five mice per strain infected with 106 IFUs of C. abortus. Experiments were performed two more times with similar results. Asterisks indicate significant differences (P < 0.01) between μMT and WT mice at the same day p.i.
C. abortus infection promotes thrombi and coagulative necrosis in μMT mice.
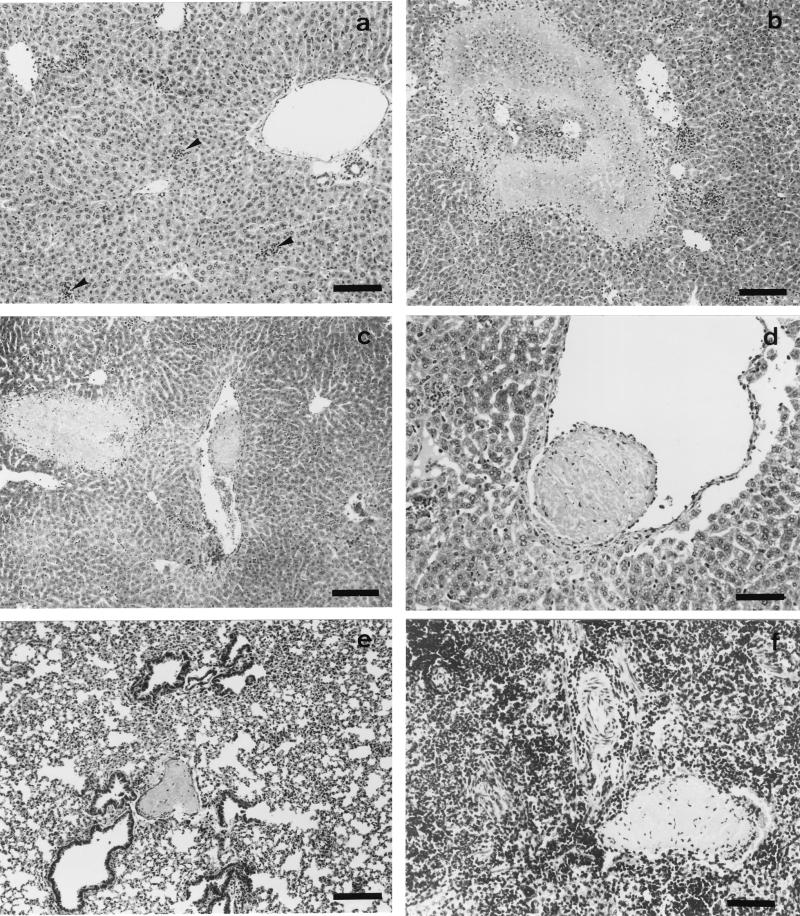
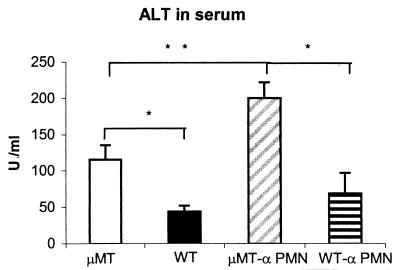
The livers, spleens, lungs, and kidneys of μMT and WT mice were examined for histopathological changes at days 2 and 4 p.i. The livers of μMT mice showed inflammatory foci with numerous neutrophils, areas of coagulative necrosis, and thrombotic lesions in the vessels at day 2 p.i. (Fig. 2b, c, and d). Thrombotic lesions were also observed in the lungs and spleens (Fig. 2e and f), although necrosis was not evident in these organs. WT mice showed no thrombi, and the only histopathological change, which was observed in these mice at day 2 p.i., was the presence of scarce inflammatory foci composed of few neutrophils (Fig. 2a). At day 4 p.i., the areas of coagulative necrosis observed in the livers of μMT mice were very large, while in the WT mice, the number and size of inflammatory foci increased but no necrosis was evident at day 4 p.i. (data not shown). In order to quantify the liver damage, the levels of ALT enzyme in serum were measured. The results showed a significantly higher level of ALT in μMT mice than in WT mice (Fig. 3).
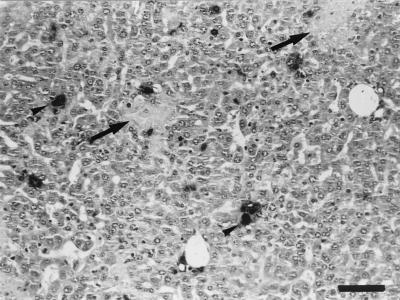
FIG. 2.
Histopathology of C. abortus-infected μMT and WT mice at day 2 p.i. Paraffin wax sections stained with hematoxylin and eosin show the disseminated intravascular coagulation in μMT mice. (a) Scarce and small inflammatory foci (arrowheads) in the livers of WT mice. (b) Area of coagulative necrosis surrounded by a substantial infiltration of neutrophils in the livers of μMT mice. (c) Thrombotic lesions near an area of coagulative necrosis in the livers of μMT mice. (d) Detail of a thrombotic lesion in the liver. (e and f) Thrombotic lesions in the lungs and spleens, respectively, of μMT mice. Original magnifications, ×100 (a, b, c, and e) and ×200 (d and f); bars, 266 μm (a, b, c, and e) and 133 μm (d and f).
FIG. 3.
Levels of the liver-associated enzyme ALT in serum. ALT levels were measured in serum taken from neutrophil-depleted and nondepleted mice at 4 days p.i. The results are expressed as means and SD from five mice per group and are representative of two separate experiments. Asterisks indicate significant differences (P < 0.01) between μMT and WT mice. The double asterisks indicate a significant difference (P < 0.01) between neutrophil-depleted and nondepleted mice of the same strain. Levels of ALT in control serum from uninfected mice were below 10 U/ml.
Infected μMT mice display a significant increase of proinflammatory cytokines in serum.
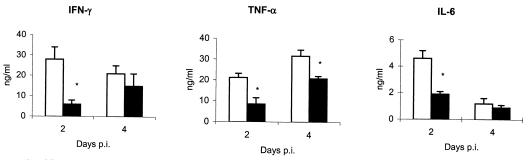
Analysis of the serum by a sandwich ELISA showed a higher level of several proinflammatory cytokines in μMT mice than in WT mice. For the three cytokines studied, IFN-γ, TNF-α, and IL-6 (Fig. 4), the main differences between μMT and WT mice were seen at day 2 p.i., coinciding with the time when thrombotic lesions in several organs were observed, as described below. At day 4 p.i., the levels of cytokines were slightly higher in μMT mice than in WT mice, although there were no significant differences except in the case of TNF-α. Analysis of the presence of these proinflammatory cytokines in liver tissue homogenates showed very similar results, with significantly higher levels of the three cytokines in μMT mice than in WT mice at day 2 p.i. (data not shown). It was impossible to culture splenocytes in μMT mice because of the high percentage of dead spleen cells.
FIG. 4.
Presence of proinflammatory cytokines in the sera of μMT (white columns) and WT (black columns) mice at 2 and 4 days p.i. The results are the means and SD for five mice. Experiments were performed two more times with similar results. Asterisks indicate significant differences (P < 0.01) between μMT and WT mice at the same day p.i.
Neutrophil depletion increases the susceptibility to C. abortus in both μMT and WT mice.
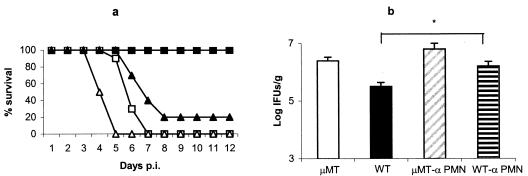
In this study, a higher number of neutrophils was observed in the very early inflammatory foci of μMT mice than in the WT mice. A similar fact has been reported in other models of infection using B-cell-deficient mice, such as infection with Leishmania donovani (39) and Francisella tularensis (2), where neutrophilia and neutrophil-mediated tissue pathology were observed. μMT mice and WT mice were depleted of neutrophils by treatment with the MAb RB6-8C5 and were infected with C. abortus to test the hypothesis that neutrophils might be responsible for the exacerbated lesions described above. Both μMT and WT mice depleted of neutrophils displayed earlier mortality than their nondepleted counterparts (Fig. 5a) and an increased number of C. abortus in the liver at day 4 p.i., with significant differences in the case of WT mice (Fig. 5b). Liver damage was evaluated in terms of the level of ALT enzyme in the sera of depleted mice. The results of this measurement showed that neutrophil depletion increased the liver damage in both mouse strains, especially in the μMT strain, where significant differences were observed, but with the significant differences between the μMT and WT mice maintained (Fig. 3). In the livers of all of the neutrophil-depleted mice, a noticeable lack of inflammatory foci was observed at day 4 p.i., although smaller areas of necrosis remained in μMT mice. The thrombotic lesions observed in the μMT mice were also less numerous than in their nondepleted counterparts. When immunohistochemical techniques were applied for detection of C. abortus antigen, numerous large, immunolabelled inclusions in hepatocytes were detected in neutrophil-depleted μMT and WT mice. In the μMT mice, the C. abortus inclusions were located next to the areas of necrosis (Fig. 6).
FIG. 5.
Evolution of C. abortus infection in μMT and WT mice depleted of neutrophils by treatment with the MAb RB6-8C5. Nondepleted μMT and WT mice served as controls for the infection study. (a) Survival of neutrophil-depleted (triangles) and nondepleted (squares) μMT (white) and WT (black) mice after infection. Groups of six mice were infected with 106 IFUs of C. abortus. Mice were monitored daily, and mortality rates were calculated. The experiment was repeated twice more, and the data shown are the results for two groups of mice of each strain (12 mice per group). (b) Quantitative isolation of C. abortus on homogenized tissues from livers collected at day 4 p.i. The number of inclusions was visualized by indirect immunofluorescence. The results are the means and SD for five mice. Experiments were performed twice with similar results. The asterisk indicates significant differences (P < 0.01) between neutrophil-depleted and nondepleted mice of the same strain.
FIG. 6.
Histopathology and immunoreaction in the livers of neutrophil-depleted μMT mice at day 4 after being infected with C. abortus. The paraffin wax section immunostained by the ABC method shows the distribution of positive immunoreaction in the chlamydial inclusions within hepatocytes (arrowheads) and areas of necrosis (arrows). Notice the lack of inflammatory foci. Original magnification, ×200; bar, 133 μm.
Neutrophil depletion alters the secretion of proinflammatory cytokines.
The presence of IFN-γ, TNF-α, and IL-6 in sera of neutrophil-depleted μMT and WT mice and their nondepleted counterparts was determined at 4 days p.i. The data showed that the depletion of neutrophils changes the relative levels of the proinflammatory cytokines (Fig. 7), with a significant decrease in some cytokines, especially TNF-α, but an increase in IL-6 in the sera of both depleted μMT and depleted WT mice.
FIG. 7.
Administration of antigranulocyte RB6-8C5 MAb during C. abortus infection alters the levels of cytokines in serum. Results shown are the mean percentages and SD of the levels of proinflammatory cytokines in the serum of neutrophil-depleted and nondepleted μMT (white columns) and WT (black columns) mice, with five mice per group. Experiments were performed twice with similar results.
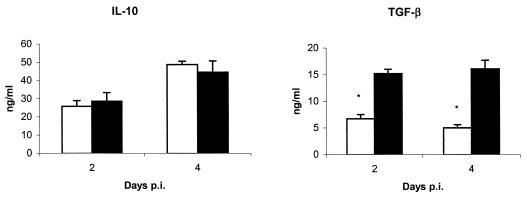
μMT mice show deficient production of the immunoregulatory cytokine TGF-β.
Since necrosis and liver damage were not completely neutrophil dependent, the presence of immunoregulatory cytokines in serum was investigated (Fig. 8). There were no significant differences in the levels of IL-10 between μMT mice and WT mice, although significantly lower levels of TGF-β were detected in the sera of μMT mice at days 2 and 4 p.i.
FIG. 8.
Presence of immunoregulatory cytokines in the sera of μMT (white columns) and WT (black columns) mice at 2 and 4 days p.i. The results are the means and SD for five mice. Experiments were performed twice with similar results. Asterisks indicate significant differences (P < 0.01) between μMT and WT mice at the same day p.i.
B-cell-deficient mice develop a delayed but efficient specific immune response to C. abortus.
It has been reported that μMT mice are unable to establish a specific response in some models of infection (29). This fact has been associated with the lack of antigen-presenting B cells in these mice. In our model, the early mortality induced by C. abortus infection in these mice prevented us from studying this important function of B cells. In order to ascertain whether μMT mice are able to establish an immune response to C. abortus, mice were infected with a sublethal dose (50-fold lower dose). At days 4 and 10 p.i., mice of both strains were killed and their spleen cells were stimulated with C. abortus antigen. μMT mice displayed a significantly lower level of IFN-γ production after specific stimulation (Table 1). There was no significant difference in the isolation of C. abortus from the two strains of mice at day 4 p.i. (data not shown). However, at day 10 p.i., the level of infection in WT mice was below the limit of detection, but μMT mice had a residual population of bacteria in the liver (3.0-log ± 0.2-log IFUs/g [mean ± standard deviation {SD}]). Six weeks after sublethal infection with C. abortus, groups of μMT and WT mice were rechallenged with a potentially lethal dose of the bacterium (106 IFUs). After the rechallenge, no signs of sickness were observed in mice of either strain. The animals were sacrificed 5 days p.i., and their spleen cells were cultured with C. abortus antigens. No differences were detected in the levels of IFN-γ production in both strains of mice (Table 1). Furthermore, isolation of C. abortus from the liver was below the limit of detection for both μMT and WT mice, confirming the establishment of an effective immune response in both strains of mice. Histopathological analysis of the liver showed no differences between μMT mice and WT mice, while scarce inflammatory foci, composed mainly of lymphocytes, were observed in the livers of both strains of mice. As a confirmation that μMT mice were indeed of the appropriate genotype, antibody levels in the sera of the reinfected mice were examined; no antibodies were detected in μMT mice, while WT mice showed higher levels of IgG2a than of IgG1 (data not shown).
TABLE 1.
Presence of IFN-γ in the supernatant of spleen cell cultures of μMT and WT mice after primary and secondary infection
| Infection (days p.i.)a | IFN-γ concn (ng/ml) in cultures stimulated with indicated antigen
|
|||
|---|---|---|---|---|
| WT
|
μMT
|
|||
| ConA | Chl-Ag | ConA | Chl-Ag | |
| Primary (10) | 18.5 ± 10.6 | 32.6 ± 5.6b | 12.8 ± 6.2 | 18.2 ± 4.4b |
| Reinfection (5) | 65.8 ± 12.6 | 48.6 ± 7.4 | 54.6 ± 8.8 | 52.4 ± 6.8 |
At the indicated time points, groups of five mice were sacrificed and spleen cell cultures were stimulated with the C. abortus antigens (Chl-Ag; 50 μg/ml) or concanavalin A (ConA; 5 μg/ml). When only medium was added to the spleen cells, no production of IFN-γ was observed. Results are expressed as means ± SD and are from one representative experiment of two independent experiments.
Significant differences (P < 0.01) between μMT and WT mice at the same day p.i.
DISCUSSION
The data presented here demonstrate that B-cell-deficient mice are highly susceptible to C. abortus infection. This increased susceptibility was based on the development of a rapid and exaggerated proinflammatory response, characterized by significantly higher levels of TNF-α, IFN-γ, and IL-6 in serum than was the case for their WT counterparts during the very early stage of infection. The existence of an endotoxin-mediated inflammatory response involving mainly TNF-α production has been previously described with the related species Chlamydia trachomatis in both in vitro (18) and in vivo (34) studies. The exacerbated proinflammatory response observed in the μMT mice in our model of infection caused multiorganic disseminated intravascular coagulation. The liver is a target for the early multiplication of C. abortus (3) and is the first organ affected by the overproduction of TNF-α (13, 24), and we observed more extensive areas of coagulative necrosis in the μMT mice. Lesions and subsequent mortality appeared only after primary infection but not when immune mice were reinfected, which suggests that the proinflammatory response was not induced by the lipopolysaccharides of the inoculum but was a consequence of the exacerbated immune response against the early multiplication of the bacteria. In contrast, μMT mice developed an effective response to reinfection after a low-level primary infection, demonstrating that when increased proinflammatory cytokine production is limited, T cells can be primed in the absence of B cells, similar to what has been described by Johansson and Lycke (20) in a genital tract model of infection with Chlamydia trachomatis.
A second main feature found in the μMT mice was an increased and earlier infiltration of neutrophils in the inflammatory foci of the liver. Neutrophilia and a deleterious effect of neutrophils in the course of infection have been reported in the infection of μMT mice by Francisella tularensis (2), and a liver neutrophil-mediated tissue pathology was observed during Leishmania donovani infection of μMT mice (39). The neutrophilia seems to be pathogen dependent in μMT mice, since in other models of infection, such as that with Mycobacterium tuberculosis, a smaller number of neutrophils was observed in the lesions (1). Our results with neutrophil-depleted μMT and WT mice indicate (i) that neutrophils are essential in the early response against C. abortus, which is in agreement with the results of previous reports (3, 32); (ii) that production of TNF-α after infection is neutrophil related in C57BL/6 mice, as has been described in other models of infection with other intracellular pathogens (28); and (iii) that thrombi and coagulative necrosis are at least in part neutrophil independent, since these features remained, although to a lesser degree, in the neutrophil-depleted μMT mice. The change in the pattern of proinflammatory cytokine production, with the increasing presence of IL-6 in the sera of depleted mice, could be explained by the secretion of proinflammatory cytokines by epithelial cells in response to the chlamydial infection, as has been described by Rasmussen et al. (35). Those authors reported the secretion of IL-6, IL-8, and granulocyte-macrophage colony-stimulating factor by chlamydia-infected epithelial cells in vitro. This result could explain the fact that in our in vivo model the level of IL-6 increased in the neutrophil-depleted mice, in which the number of infected hepatocytes was higher.
We suggest that an exacerbated proinflammatory response mediated by both neutrophils and hepatocytes caused the disseminated intravascular coagulation that led to the deaths of the μMT mice. The response might be induced by the higher burden of C. abortus found in the liver, probably due to the lack of APC function of B cells, as has been described for Chlamydia trachomatis by Yang and Brunham (44). However, a higher burden of C. abortus has been detected in other models of infection in C57BL/6 mice, such as natural killer cell depletion (our unpublished data) or infection with a lethal dose in WT mice (10-fold higher than the dose used in this study), with no noticeable exaggerated proinflammatory response or thrombi. Furthermore, when a low, sublethal dose was used, there was no difference in the burdens of C. abortus in the livers of μMT and WT mice at day 4 p.i., suggesting that B cells are not essential to the control of the multiplication of C. abortus but could have some role in the control of the inflammatory response induced by the high infective dose. Differences found with the lethal dose might be caused by the multiorganic failure associated with disseminated intravascular coagulation. Indeed, it was impossible to culture splenocytes in μMT mice because of the high percentage of dead spleen cells. Since similar extensive necrotic lesions have been described in several models of infection where a lack of regulation in the immune response was reported (14, 15, 17, 27), an interesting hypothesis raised by our study is that B cells might play an immunoregulatory function in our model of C. abortus infection and that the lack of B cells leads to the uncontrolled response. The finding of significantly lower levels of TGF-β in μMT mice supports this theory, since this cytokine is a potent immunosuppressor molecule in some conditions (25). Recently, it has been reported that B cells play an important immunoregulatory role in the maintenance of the tolerance and prevention of autoimmune diseases by producing TGF-β (16, 42, 43). Especially interesting for our study is the report of Tian et al. (42), who found that lipopolysaccharide-activated B cells are able to down-regulate the IFN-γ response by producing TGF-β. A similar situation may have occurred in our model, where B-cell-deficient mice, defective in TGF-β production, were unable to appropriately regulate the early inflammatory response of macrophages, neutrophils, and infected cells. Thrombi and death from shock would have happened before T-cell-mediated immunity had an opportunity to intervene.
Acknowledgments
We thank R. L. Coffman for the generous gift of RB6-8C5 hybridoma cells.
This work was partially supported by a grant from the Ministerio Ciencia y Tecnología (AGL2001-0627). L. Del Río and N. Ortega were the recipients of predoctoral grants from the Ministerio Ciencia y Tecnología and Universidad de Murcia, respectively.
Editor: J. D. Clements
REFERENCES
- 1.Bosio, C. M., D. Gradner, and K. L. Elkins. 2000. Infection of B cell-deficient mice with CDC 1551, a clinical isolate of Mycobacterium tuberculosis: delay in dissemination and development of lung pathology. J. Immunol. 164:6417-6425. [DOI] [PubMed] [Google Scholar]
- 2.Bosio, C. M., and K. L. Elkins. 2001. Susceptibility to secondary Francisella tularensis live vaccine strain infection in B-cell-deficient mice is associated with neutrophilia but not with defects in specific T-cell-mediated immunity. Infect. Immun. 69:194-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buendía, A. J., R. Montes de Oca, J. A. Navarro, J. Sánchez, F. Cuello, and J. Salinas. 1999. Role of polymorphonuclear neutrophils in a murine model of Chlamydia psittaci-induced abortion. Infect. Immun. 67:2110-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buendía, A. J., J. Salinas, J. Sánchez, M. C. Gallego, A. Rodolakis, and F. Cuello. 1997. Localisation by immunoelectron microscopy of antigens of Chlamydia psittaci suitable for diagnosis or vaccine development. FEMS Microbiol. Lett. 150:113-119. [DOI] [PubMed] [Google Scholar]
- 5.Buendía, A. J., J. Sánchez, L. Del Río, B. Garcés, M. C. Gallego, M. R. Caro, A. Bernabé, and J. Salinas. 1999. Differences in the immune response against ruminant chlamydial strains in a murine model. Vet. Res. 30:495-507. [PubMed] [Google Scholar]
- 6.Buendía, A. J., J. Sánchez, M. C. Martínez, P. Cámara, J. A. Navarro, A. Rodolakis, and J. Salinas. 1998. Kinetics of infection and effects on placental cell populations in a murine model of Chlamydia psittaci-induced abortion. Infect. Immun. 66:2128-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzoni-Gatel, D., L. Guilloteau, F. Bernard, S. Bernard, T. Chardes, and A. Rocca. 1992. Protection against Chlamydia psittaci in mice conferred by Lyt-2+ T cells. Immunology 77:284-288. [PMC free article] [PubMed] [Google Scholar]
- 8.Culkin, S. J., T. Rhinehart-Jones, and K. L. Elkins. 1997. A novel role for B cells in early protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J. Immunol. 158:3277-3284. [PubMed] [Google Scholar]
- 9.Del Río, L., A. J. Buendía, J. Sánchez, M. C. Gallego, M. R. Caro, N. Ortega, J. Seva, F. J. Pallares, F. Cuello, and J. Salinas. 2001. Endogenous interleukin-12 is not required for resolution of Chlamydophila abortus (Chlamydia psittaci serotype 1) infection in mice. Infect. Immun. 69:4808-4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Río, L., A. J. Buendía, J. Sánchez, B. Garcés, M. R. Caro, M. C. Gallego, A. Bernabé, F. Cuello, and J. Salinas. 2000. Chlamydophila abortus (Chlamydia psittaci serotype 1) clearance is associated with the early recruitment of neutrophils and CD8+ T cells in a mouse model. J. Comp. Pathol. 123:171-181. [DOI] [PubMed] [Google Scholar]
- 11.De Sa, C., A. Souriau, F. Bernard, J. Salinas, and A. Rodolakis. 1995. An oligomer of the major outer membrane protein of Chlamydia psittaci is recognized by monoclonal antibodies which protect mice from abortion. Infect. Immun. 63:4912-4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshpande, S. P., U. Kumaraguru, and B. T. Rouse. 2000. Dual role of B cells in mediating innate and acquired immunity to herpes simplex virus infections. Cell. Immunol. 202:79-87. [DOI] [PubMed] [Google Scholar]
- 13.Esmon, C. T., K. Fukudome, T. Mather, W. Bode, L. M. Regan, D. J. Stearns-Kurosawa, and S. Kurosawa. 1999. Inflammation, sepsis, and coagulation. Haematologica 84:254-259. [PubMed] [Google Scholar]
- 14.Fallon, P. G., and D. W. Dunne. 1999. Toleration of mice to Schistosoma mansoni egg antigens causes elevated type 1 and diminished type 2 cytokine responses and increased mortality in acute infection. J. Immunol. 162:4122-4132. [PubMed] [Google Scholar]
- 15.Gazzinelli, R. T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kühn, W. Müller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J. Immunol. 157:798-805. [PubMed] [Google Scholar]
- 16.Gonnella, P. A., H. P. Waldner, and H. L. Weiner. 2001. B cell-deficient (μMT) mice have alterations in the cytokine microenvironment of the gut-associated lymphoid tissue (GALT) and a defect in the low dose mechanism of oral tolerance. J. Immunol. 166:4456-4464. [DOI] [PubMed] [Google Scholar]
- 17.Holscher, C., M. Mohrs, W. J. Dai, G. Kohler, B. Ryffel, G. A. Schaub, H. Mossmann, and F. Brombacher. 2000. Tumor necrosis factor alpha-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infect. Immun. 68:4075-4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingalls, R. R., P. A. Rice, N. Qureshi, K. Takayama, J. S. Lin, and D. T. Golenbock. 1995. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect. Immun. 63:3125-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankovic, D., A. W. Cheever, M. C. Kulberg, T. A. Wynn, G. Yap, P. Caspar, F. A. Lewis, R. Clynes, J. V. Ravetch, and A. Sher. 1998. CD4+ T cell-mediated granulomatous pathology in schistosomiasis is downregulated by a B cell-dependent mechanism requiring Fc receptor signaling. J. Exp. Med. 187:619-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson, M., and N. Lycke. 2001. Immunological memory in B-cell-deficient mice conveys long-lasting protection against genital tract infection with Chlamydia trachomatis by rapid recruitment of T cells. Immunology 102:199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitamura, D., J. Roes, R. Khun, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 22.Kurt-Jones, E. A., D. Liano, K. A. HayGlass, B. Benacerraf, M. S. Sy, and A. K. Abbas. 1988. The role of antigen-presenting B cells in T cell priming in vivo: studies of B cell-deficient mice. J. Immunol. 140:3773-3778. [PubMed] [Google Scholar]
- 23.Leef, M., K. L. Elkins, J. Barbic, and R. D. Shahin. 2000. Protective immunity to Bordetella pertussis requires both B cells and CD4+ T cells for key functions other than specific antibody production. J. Exp. Med. 191:1841-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leist, M., F. Gantner, I. I. Bohlinger, G. Tiegs, P. G. Germann, and A. Wendel. 1995. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am. J. Pathol. 146:1220-1234. [PMC free article] [PubMed] [Google Scholar]
- 25.Letterio, J. J., and A. B. Robert. 1998. Regulation of immune responses by TGF-β. Annu. Rev. Immunol. 16:137-161. [DOI] [PubMed] [Google Scholar]
- 26.Mahon, B. P., B. J. Sheahan, F. Griffin, G. Murphy, and K. H. Mills. 1997. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-γ receptor or immunoglobulin μ chain genes. J. Exp. Med. 186:1843-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall, A. J., L. R. Brunet, Y. van Gessel, A. Alcaraz, S. K. Bliss, E. J. Pearce, and E. Y. Denkers. 1999. Toxoplasma gondii and Schistosoma mansoni synergize to promote hepatocyte dysfunction associated with high levels of plasma TNF-α and early death in C57BL/6 mice. J. Immunol. 163:2089-2097. [PubMed] [Google Scholar]
- 28.Marshall, A. J., and E. Y. Denkers. 1998. Toxoplasma gondii triggers granulocyte-dependent cytokine-mediated lethal shock in d-galactosamine-sensitized mice. Infect. Immun. 66:1325-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuzaki, G., H. M. Vordermeier, A. Hashimoto, K. Nomoto, and J. Ivanyi. 1999. The role of B cells in the establishment of T cell response in mice infected with an intracellular bacteria, Listeria monocytogenes. Cell. Immunol. 194:178-185. [DOI] [PubMed] [Google Scholar]
- 30.McCafferty, M. C., S. W. Maley, G. Entrican, and D. Buxton. 1994. The importance of interferon-γ in an early infection of Chlamydia psittaci in mice. Immunology 81:631-636. [PMC free article] [PubMed] [Google Scholar]
- 31.Mittrücker, H. W., B. Raupach, A. Kholer, and S. H. E. Kaufmann. 2000. Cutting edge: role of B lymphocytes in protective immunity against Salmonella typhimurium infection. J. Immunol. 164:1648-1652. [DOI] [PubMed] [Google Scholar]
- 32.Montes de Oca, R., A. J. Buendía, L. Del Río, J. Sánchez, J. Salinas, and J. A. Navarro. 2000. Polymorphonuclear neutrophils are necessary for the recruitment of CD8+ T cells in the liver in a pregnant mouse model of Chlamydophila abortus (Chlamydia psittaci serotype 1) infection. Infect. Immun. 68:1746-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perfettini, J. L., T. Darville, G. Gachelin, P. Souque, M. Huerre, A. Dautry-Varsat, and D. M. Ojcius. 2000. Effect of Chlamydia trachomatis infection and subsequent tumor necrosis factor alpha secretion on apoptosis in the murine genital tract. Infect. Immun. 68:2237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasmussen, S. J., L. Eckmann, A. J. Quayle, L. Shen, Y. X. Zhang, D. J. Anderson, J. Fierer, R. S. Stephens, and M. F. Kagnoff. 1997. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Investig. 99:77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodolakis, A., J. Salinas, and J. Papp. 1998. Recent advances on ovine chlamydial abortion. Vet. Res. 29:275-288. [PubMed] [Google Scholar]
- 37.Salinas, J., A. Souriau, F. Cuello, and A. Rodolakis. 1995. Antigenic diversity of ruminant Chlamydia psittaci strains demonstrated by the indirect microimmunofluorescent test with monoclonal antibodies. Vet. Microbiol. 43:219-226. [DOI] [PubMed] [Google Scholar]
- 38.Sayles, P. C., G. W. Gibson, and L. L. Johnson. 2000. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect. Immun. 68:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smelt, S. C., S. E. J. Cotterell, C. R. Engwerda, and P. M. Kaye. 2000. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J. Immunol. 164:3681-3688. [DOI] [PubMed] [Google Scholar]
- 40.Su, H., K. Felzer, H. D. Caldwell, and R. P. Morrison. 1997. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect. Immun. 65:1993-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tepper, R. I., R. L. Coffman, and P. Leder. 1992. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science 257:548-551. [DOI] [PubMed] [Google Scholar]
- 42.Tian, J., D. Zekzer, L. Hanssen, Y. Lu, A. Olcott, and D. L. Kaufman. 2001. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in non-obese diabetic mice. J. Immunol. 167:1081-1089. [DOI] [PubMed] [Google Scholar]
- 43.Valujskikh, A., A. M. VanBuskirk, C. G. Orosz, and P. S. Heeger. 2001. A role for TGFβ and B cells in immunologic tolerance after intravenous injection of soluble antigen. Transplantation 72:685-693. [DOI] [PubMed] [Google Scholar]
- 44.Yang, X., and R. C. Brunham. 1998. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J. Immunol. 161:1439-1446. [PubMed] [Google Scholar]