Abstract
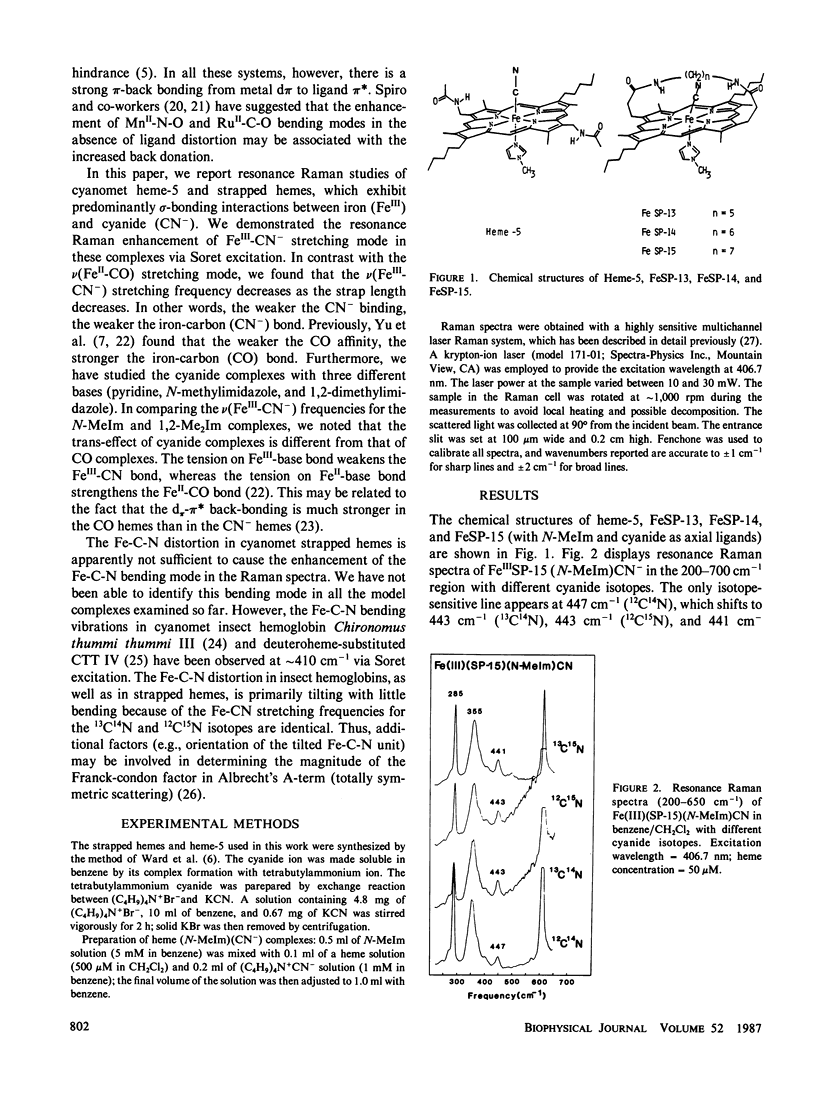
We report resonance Raman studies of the iron-carbon bond stretching vibrations, nu(Fe-CN), in sterically hindered and unhindered heme (FeIII)-CN- complexes. The sterically hindred "strapped hemes" are equipped with a covalently linked 13-, 14-, or 15-atom hydrocarbon chain across one face of the heme; these are called FeSP-13, FeSP-14, and FeSP-15, respectively. These straps would presumably exert a sideway shearing strain to force the linear ligands (e.g., CN- and CO) to be tilted and/or bent. The shorter the chain length, the weaker the ligand binding affinity because of a greater steric hindrance. This study reveals that the nu(Fe-CN) frequency decreases as the chain length is decreased, in contrast with the CO complexes, where the nu(Fe-CO) frequency increases as the chain length is decreased. For the heme-CN- complexes (with N-methylimidazole as a base), the nu(Fe-CN) frequencies are: heme 5 (unhindered), 451 cm-1; FeSP-15, 447 cm-1; FeSP-14, 447 cm-1; FeSP-13, 445 cm-1. For the heme-CO complexes (with N-methylimidazole as a base), the nu(Fe-CO) frequencies are: heme 5, 495 cm-1; FeSP-15, 509 cm-1; FeSP-14, 512 cm-1; FeSP-13, 514 cm-1 (Yu, N.-T., E. A. Kerr, B. Ward, and C. K. Chang, 1983, Biochemistry, 22:4534-4540). We have also studied the cyanide complexes with three different bases (pyridine, N-methylimidazole and 1,2-dimethylimidazole), and found that the trans-effect of cyanide complex is different from that of CO complexes. The tension on Fe"'-base bond weakens the Fe"'-CN- bond, whereas the tension on Fe"-base bond strengthens the Fe"-CO bond. The origin of these differences may be attributed to different extents of the d7r(Fe)- wr*(ligand) back bonding between the CN- and CO heme complexes. The Fe-C-N bending vibrations in these cyanomet strapped hemes are not resonance-enhanced, although this bending mode has been detected at -410 cm-' via Soret excitation in cyanometinsect hemoglobins. It is suggested that the orientation of the tilted Fe-C-N unit may be important in determining the overlap between CN and porphyrin 7r* orbitals, which provide coupling of the Fe-C-N bending mode with the resonant Soret (r-7r*) transition.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benko B., Yu N. T. Resonance Raman studies of nitric oxide binding to ferric and ferrous hemoproteins: detection of Fe(III)--NO stretching, Fe(III)--N--O bending, and Fe(II)--N--O bending vibrations. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7042–7046. doi: 10.1073/pnas.80.22.7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson S. D., Constantinidis I., Satterlee J. D., Ondrias M. R. A resonance Raman study of ligand binding geometry in Glycera dibranchiata carbonmonoxyhemoglobin. J Biol Chem. 1985 Jul 25;260(15):8741–8745. [PubMed] [Google Scholar]
- Caughey W. S. Carbon monoxide bonding in hemeproteins. Ann N Y Acad Sci. 1970 Oct 5;174(1):148–153. doi: 10.1111/j.1749-6632.1970.tb49781.x. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Doxsee K. M. Carbon monoxide binding to iron porphyrins. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6035–6039. doi: 10.1073/pnas.76.12.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Halbert T. R., Suslick K. S. Nature of O2 and CO binding to metalloporphyrins and heme proteins. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3333–3337. doi: 10.1073/pnas.73.10.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista-Kirkup R., Smulevich G., Spiro T. G. Alternative carbon monoxide binding modes for horseradish peroxidase studied by resonance Raman spectroscopy. Biochemistry. 1986 Jul 29;25(15):4420–4425. doi: 10.1021/bi00363a037. [DOI] [PubMed] [Google Scholar]
- Kerr E. A., Mackin H. C., Yu N. T. Resonance Raman studies of carbon monoxide binding to iron "picket fence" porphyrin with unhindered and hindered axial bases. An inverse relationship between binding affinity and the strength of iron-carbon bond. Biochemistry. 1983 Sep 13;22(19):4373–4379. doi: 10.1021/bi00288a005. [DOI] [PubMed] [Google Scholar]
- Kerr E. A., Yu N. T., Bartnicki D. E., Mizukami H. Resonance Raman studies of CO and O2 binding to elephant myoglobin (distal His(E7)----Gln). J Biol Chem. 1985 Jul 15;260(14):8360–8365. [PubMed] [Google Scholar]
- Kitagawa T., Kyogoku Y., Iizuka T., Saito M. I. Nature of the iron-ligand bond in ferrous low spin hemoproteins studied by resonance Raman scattering. J Am Chem Soc. 1976 Aug 18;98(17):5169–5173. doi: 10.1021/ja00433a019. [DOI] [PubMed] [Google Scholar]
- Peng S. M., Ibers J. A. Stereochemistry of carbonylmetalloporphyrins. The structure of (pyridine)(carbonyl)(5, 10, 15, 20-tetraphenylprophinato)iron(II). J Am Chem Soc. 1976 Dec 8;98(25):8032–8036. doi: 10.1021/ja00441a025. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Structure and mechanism of haemoglobin. Br Med Bull. 1976 Sep;32(3):195–208. doi: 10.1093/oxfordjournals.bmb.a071363. [DOI] [PubMed] [Google Scholar]
- Rousseau D. L., Ondrias M. R., LaMar G. N., Kong S. B., Smith K. M. Resonance Raman spectra of the heme in leghemoglobin. Evidence for the absence of ruffling and the influence of the vinyl groups. J Biol Chem. 1983 Feb 10;258(3):1740–1746. [PubMed] [Google Scholar]
- Smulevich G., Evangelista-Kirkup R., English A., Spiro T. G. Raman and infrared spectra of cytochrome c peroxidase-carbon monoxide adducts in alternative conformational states. Biochemistry. 1986 Jul 29;25(15):4426–4430. doi: 10.1021/bi00363a038. [DOI] [PubMed] [Google Scholar]
- Tsubaki M., Srivastava R. B., Yu N. T. Resonance Raman investigation of carbon monoxide bonding in (carbon monoxy)hemoglobin and -myoglobin: detection of Fe-CO stretching and Fe-C-O bending vibrations and influence of the quaternary structure change. Biochemistry. 1982 Mar 16;21(6):1132–1140. doi: 10.1021/bi00535a004. [DOI] [PubMed] [Google Scholar]
- Uno T., Nishimura Y., Makino R., Iizuka T., Ishimura Y., Tsuboi M. The resonance Raman frequencies of the Fe-CO stretching and bending modes in the CO complex of cytochrome P-450cam. J Biol Chem. 1985 Feb 25;260(4):2023–2026. [PubMed] [Google Scholar]
- Uno T., Nishimura Y., Tsuboi M., Kita K., Anraku Y. Resonance Raman study of cytochrome b562-o complex, a terminal oxidase of Escherichia coli in its ferric, ferrous, and CO-ligated states. J Biol Chem. 1985 Jun 10;260(11):6755–6760. [PubMed] [Google Scholar]
- Yu N. T., Benko B., Kerr E. A., Gersonde K. Iron-carbon bond lengths in carbonmonoxy and cyanomet complexes of the monomeric hemoglobin III from Chironomus thummi thummi: a critical comparison between resonance Raman and x-ray diffraction studies. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5106–5110. doi: 10.1073/pnas.81.16.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N. T., Kerr E. A., Ward B., Chang C. K. Resonance Raman detection of Fe-CO stretching and Fe-C-O bending vibrations in sterically hindered carbonmonoxy "strapped hemes". A structural probe of Fe-C-O distortion. Biochemistry. 1983 Sep 13;22(19):4534–4540. doi: 10.1021/bi00288a028. [DOI] [PubMed] [Google Scholar]
- Yu N. T. Resonance Raman studies of ligand binding. Methods Enzymol. 1986;130:350–409. doi: 10.1016/0076-6879(86)30018-1. [DOI] [PubMed] [Google Scholar]


