Abstract
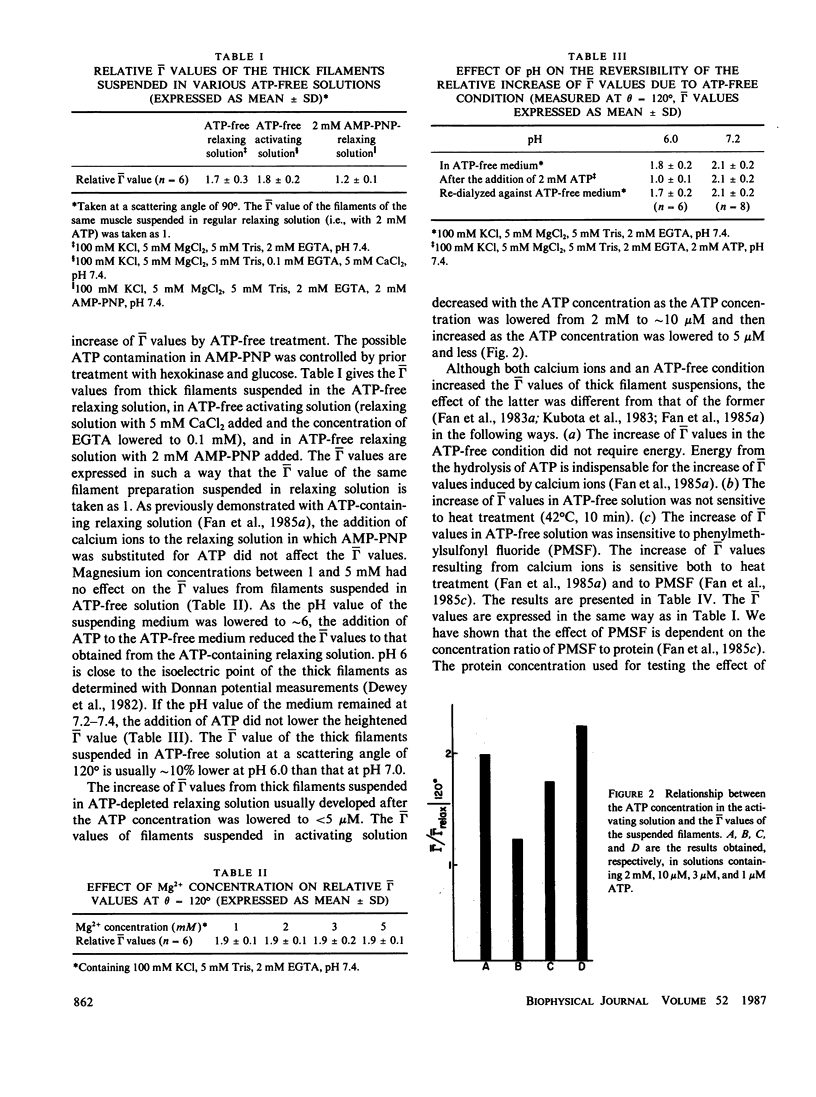
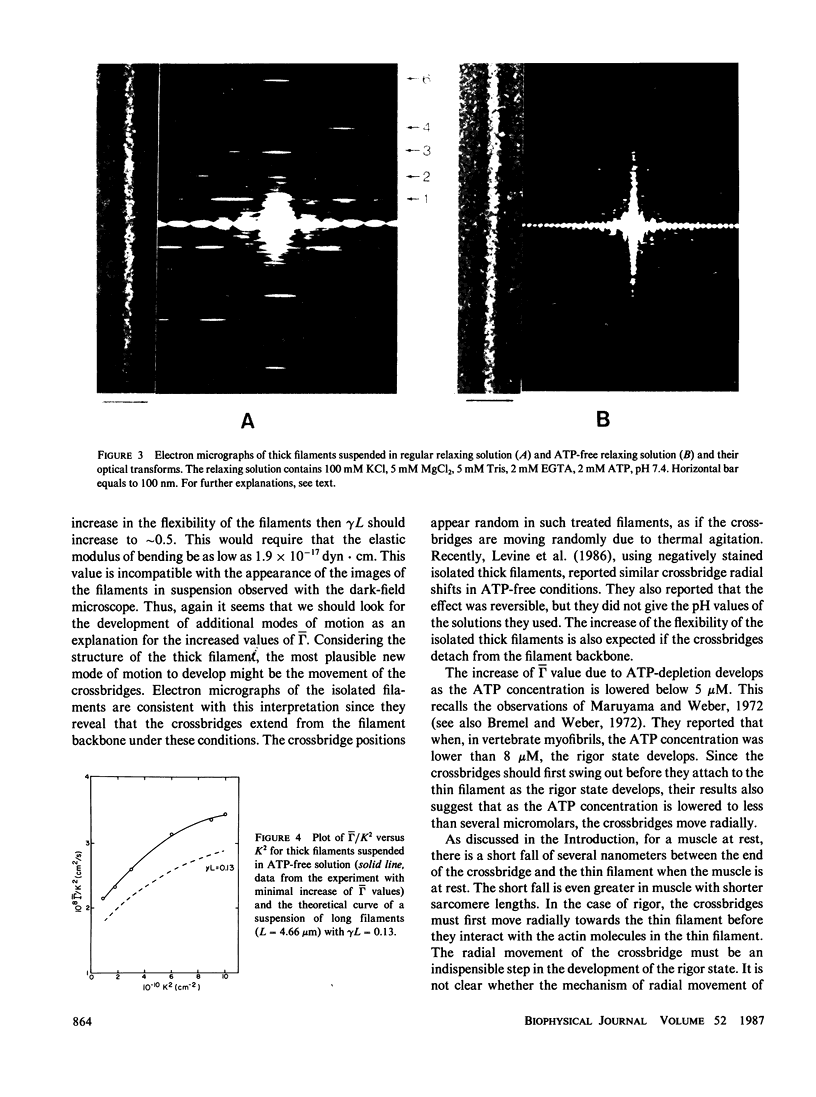
With the dynamic light scattering method, we have shown that calcium ions increase the high-frequency internal motions of isolated thick filaments of Limulus striated muscle in the presence of ATP (Kubota, K., B. Chu, Shih-fang Fan, M.M. Dewey, P. Brink, and D. Colflesh. J. Mol. Biol. 1983. 166:329 and Fan, S.-f., M.M. Dewey, D. Colflesh, B. Gaylinn, R. Greguski, and B. Chu. Biophys. J. 1985. 47:809). If ATP is removed from the suspending medium, an increase of high-frequency internal motions also has been observed with characteristics different from those of filaments suspended in a medium containing ATP and calcium ions. These internal motions appear whether calcium ions are present or not and are suppressed by trifluoperazine (TFP). The motions differ from the calcium ion-induced motions in that (a) an energy supply is not required; (b) they are insensitive to heat treatment (42 degrees C, 10 min) and (c) they are also insensitive to phenylmethylsulfonyl fluoride which blocks the motions in the presence of ATP and calcium ions (Fan, Shih-fang, M.M. Dewey, D. Colflesh, B. Gaylinn, R. Greguski, and B. Chu. 1985. Biochim. Biophys. Acta. 827:101). Electron micrographs of negatively stained thick filaments in an ATP-free medium show that the majority of crossbridges extend out from the backbone of the filament and optical diffraction patterns from these filaments lack layer lines arising from the crossbridges. The flexibility of the thick filaments suspended in ATP-free media increases.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burghardt T. P., Ando T., Borejdo J. Evidence for cross-bridge order in contraction of glycerinated skeletal muscle. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7515–7519. doi: 10.1073/pnas.80.24.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Fan S. F., Dewey M. M., Colflesh D., Brink P., Chu B. Dynamic laser light scattering of papain-treated thick filaments from limulus striated muscle in suspension. Adv Exp Med Biol. 1984;170:89–92. doi: 10.1007/978-1-4684-4703-3_8. [DOI] [PubMed] [Google Scholar]
- Fan S. F., Dewey M. M., Colflesh D., Gaylinn B., Greguski R. A., Chu B. The active cross-bridge motions of isolated thick filaments from myosin-regulated muscles detected by quasi-elastic light scattering. Biophys J. 1985 Jun;47(6):809–821. doi: 10.1016/S0006-3495(85)83985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S. F., Dewey M. M., Colflesh D., Gaylinn B., Greguski R., Chu B. Suppression of active cross-bridge motions of isolated thick myofilaments in suspension by phenylmethylsulfonyl fluoride. Biochim Biophys Acta. 1985 Jan 21;827(1):101–105. doi: 10.1016/0167-4838(85)90105-0. [DOI] [PubMed] [Google Scholar]
- Fan S. F., Dewey M. M., Colflesh D. The flexibility of isolated limulus thick filaments in relaxed and activated States. Biophys J. 1985 Nov;48(5):859–861. doi: 10.1016/S0006-3495(85)83846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J Mol Biol. 1975 Feb 15;92(1):113–143. doi: 10.1016/0022-2836(75)90094-7. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. Structural difference between resting and rigor muscle; evidence from intensity changes in the lowangle equatorial x-ray diagram. J Mol Biol. 1968 Nov 14;37(3):507–520. doi: 10.1016/0022-2836(68)90118-6. [DOI] [PubMed] [Google Scholar]
- Ip W., Heuser J. Direct visualization of the myosin crossbridge helices on relaxed rabbit psoas thick filaments. J Mol Biol. 1983 Nov 25;171(1):105–109. doi: 10.1016/s0022-2836(83)80317-9. [DOI] [PubMed] [Google Scholar]
- Kubota K., Chu B., Fan S. F., Dewey M. M., Brink P., Colflesh D. E. Quasi-elastic light scattering of suspensions of Limulus thick myofilaments in relaxed (long) activated and rerelaxed (short) states. J Mol Biol. 1983 May 25;166(3):329–340. doi: 10.1016/s0022-2836(83)80088-6. [DOI] [PubMed] [Google Scholar]
- Levine R. J., Kensler R. W., Levitt P. Crossbridge and backbone structure of invertebrate thick filaments. Biophys J. 1986 Jan;49(1):135–138. doi: 10.1016/S0006-3495(86)83624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy H. M., Ryan E. M. The contractile and control sites of natural actomyosin. J Gen Physiol. 1967 Nov;50(10):2421–2435. doi: 10.1085/jgp.50.10.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Rodger C. D., Tregear R. T. Changes in muscle crossbridges when beta, gamma-imido-ATP binds to myosin. J Mol Biol. 1976 Jun 14;104(1):263–276. doi: 10.1016/0022-2836(76)90012-7. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Weber A. Binding of adenosine triphosphate to myofibrils during contraction and relaxation. Biochemistry. 1972 Aug 1;11(16):2990–2998. doi: 10.1021/bi00766a010. [DOI] [PubMed] [Google Scholar]
- Nagashima H., Asakura S. Dark-field light microscopic study of the flexibility of F-actin complexes. J Mol Biol. 1980 Jan 15;136(2):169–182. doi: 10.1016/0022-2836(80)90311-3. [DOI] [PubMed] [Google Scholar]
- Newman J., Swinney H. L., Day L. A. Hydrodynamic properties and structure of fd virus. J Mol Biol. 1977 Nov 5;116(3):593–603. doi: 10.1016/0022-2836(77)90086-9. [DOI] [PubMed] [Google Scholar]
- Padrón R., Huxley H. E. The effect of the ATP analogue AMPPNP on the structure of crossbridges in vertebrate skeletal muscles: X-ray diffraction and mechanical studies. J Muscle Res Cell Motil. 1984 Dec;5(6):613–655. doi: 10.1007/BF00713923. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida T. Angles of nucleotides bound to cross-bridges in glycerinated muscle fiber at various concentrations of epsilon-ATP, epsilon-ADP and epsilon-AMPPNP detected by polarized fluorescence. J Mol Biol. 1981 Mar 15;146(4):539–560. doi: 10.1016/0022-2836(81)90046-2. [DOI] [PubMed] [Google Scholar]