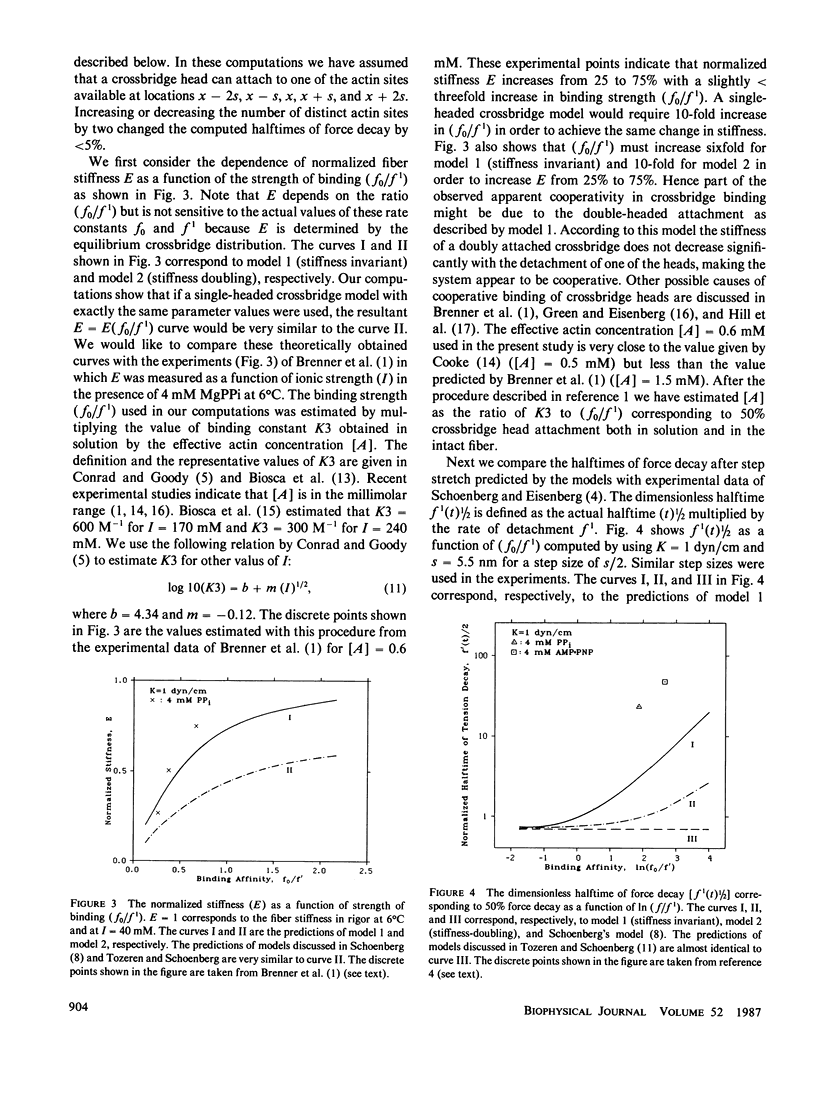
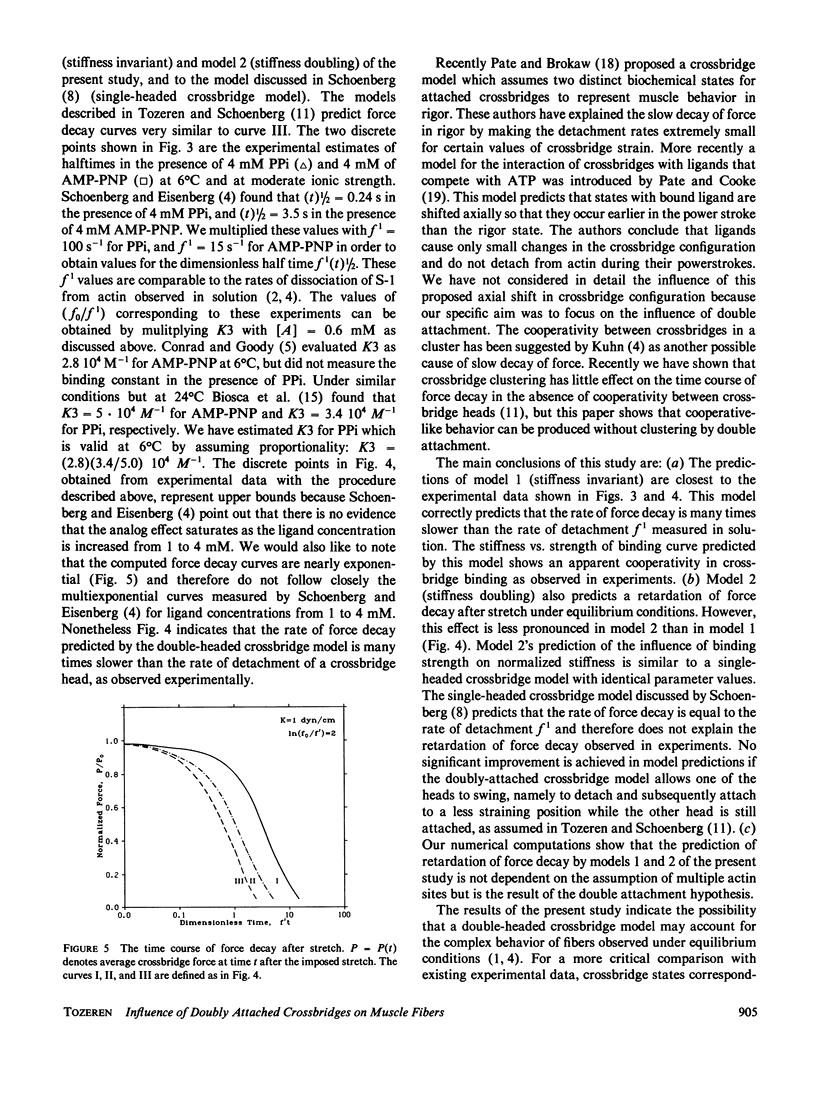
Abstract
A simple model of a double-headed crossbridge is introduced to explain the retardation of force decay after an imposed stretch in skeletal muscle fibers under equilibrium conditions. The critical assumption in the model is that once one of the heads of a crossbridge is attached to one of the available actin sites, the attachment of the second head will be restricted to a level of strain determined by the attachment of the first head. The crossbridge structure, namely the connection of both heads of a crossbridge to the same tail region, is assumed to impose this constraint on the spatial configurations of crossbridge heads. The unique feature of the model is the prediction that, in the presence of a ligand (PPi, ADP, AMP-PNP) and absence of Ca2+, the halftime of force decay is many times larger than the inverse rate of detachment of a crossbridge head measured in solution. This prediction is in agreement with measured values of half-times of force decay in fibers under similar conditions (Schoenberg, M., and E. Eisenberg. 1985. Biophys. J. 48:863-871f). It is predicted that a crossbridge head is more likely to re-attach to its previously strained position than remain unattached while the other head is attached, leading to the slow decay of force. Our computations also show that the apparent cooperativity in crossbridge binding observed in experiments (Brenner, B., L. C. Yu, L. E. Greene, E. Eisenberg, and M. Schoenberg. 1986. Biophys. J. 50:1101-1108) can be partially accounted by the double-headed crossbridge attachment. Our model predictions fit the aforementioned data best when the crossbridge stiffness does not change significantly with the dissociation of one of the two attached heads. This observation suggests that crossbridge stiffness is determined either by the extensibility (flexibility) of the double helical tail region or its junction to the thick filament backbone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biosca J. A., Greene L. E., Eisenberg E. Binding of ADP and ATP analogs to cross-linked and non-cross-linked acto X S-1. J Biol Chem. 1986 Jul 25;261(21):9793–9800. [PubMed] [Google Scholar]
- Borejdo J., Oplatka A. Heavy meromyosin cross-links thin filaments in striated muscle myofibrils. Nature. 1981 May 28;291(5813):322–323. doi: 10.1038/291322a0. [DOI] [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Greene L. E., Eisenberg E., Schoenberg M. Ca2+-sensitive cross-bridge dissociation in the presence of magnesium pyrophosphate in skinned rabbit psoas fibers. Biophys J. 1986 Dec;50(6):1101–1108. doi: 10.1016/S0006-3495(86)83554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M. L., Tregear R. T. Tension maintenance and crossbridge detachment. FEBS Lett. 1982 Jul 5;143(2):217–219. doi: 10.1016/0014-5793(82)80102-6. [DOI] [PubMed] [Google Scholar]
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Formation of a ternary complex: actin, 5'-adenylyl imidodiphosphate, and the subfragments of myosin. Proc Natl Acad Sci U S A. 1978 Jan;75(1):54–58. doi: 10.1073/pnas.75.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Theoretical formalism for the sliding filament model of contraction of striated muscle. Part II. Prog Biophys Mol Biol. 1975;29(2):105–159. doi: 10.1016/0079-6107(76)90021-3. [DOI] [PubMed] [Google Scholar]
- Konrad M., Goody R. S. Kinetic and thermodynamic properties of the ternary complex between F-actin, myosin subfragment 1 and adenosine 5'-[beta, gamma-imido]triphosphate. Eur J Biochem. 1982 Nov 15;128(2-3):547–555. doi: 10.1111/j.1432-1033.1982.tb07000.x. [DOI] [PubMed] [Google Scholar]
- Kuhn H. J. Cross bridge slippage induced by the ATP analogue AMP-PNP and stretch in glycerol-extracted fibrillar muscle fibres. Biophys Struct Mech. 1978 Apr 13;4(2):159–168. doi: 10.1007/BF00539229. [DOI] [PubMed] [Google Scholar]
- Marston S. B. The rates of formation and dissociation of actin-myosin complexes. Effects of solvent, temperature, nucleotide binding and head-head interactions. Biochem J. 1982 May 1;203(2):453–460. doi: 10.1042/bj2030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E. F., Brokaw C. J. Cross-bridge behavior in rigor muscle. Biophys Struct Mech. 1980;7(1):51–63. doi: 10.1007/BF00538158. [DOI] [PubMed] [Google Scholar]
- Pate E., Cooke R. The inhibition of muscle contraction by adenosine 5' (beta, gamma-imido) triphosphate and by pyrophosphate. Biophys J. 1985 Jun;47(6):773–780. doi: 10.1016/S0006-3495(85)83980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M., Eisenberg E. Muscle cross-bridge kinetics in rigor and in the presence of ATP analogues. Biophys J. 1985 Dec;48(6):863–871. doi: 10.1016/S0006-3495(85)83847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M. Equilibrium muscle cross-bridge behavior. Theoretical considerations. Biophys J. 1985 Sep;48(3):467–475. doi: 10.1016/S0006-3495(85)83802-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tözeren A., Schoenberg M. The effect of cross-bridge clustering and head-head competition on the mechanical response of skeletal muscle under equilibrium conditions. Biophys J. 1986 Nov;50(5):875–884. doi: 10.1016/S0006-3495(86)83528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Rigor contraction and the effect of various phosphate compounds on glycerinated insect flight and vertebrate muscle. J Physiol. 1970 Jul;208(3):583–605. doi: 10.1113/jphysiol.1970.sp009138. [DOI] [PMC free article] [PubMed] [Google Scholar]


