Abstract
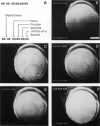
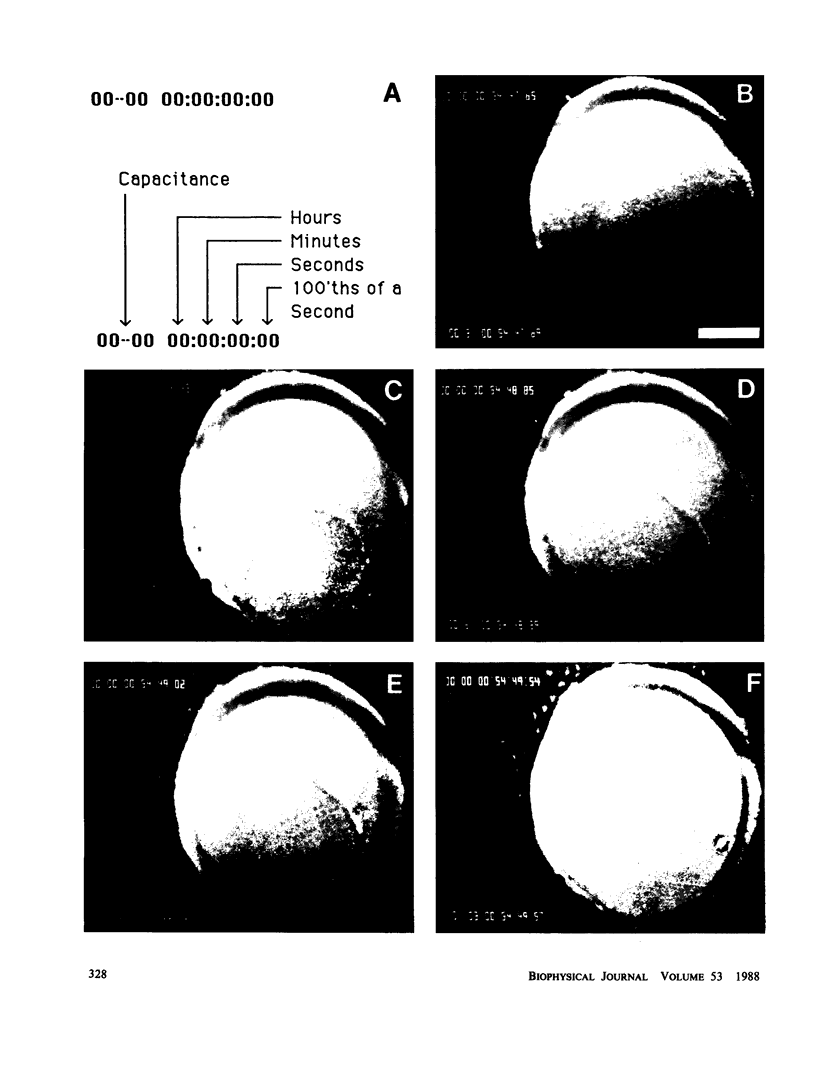
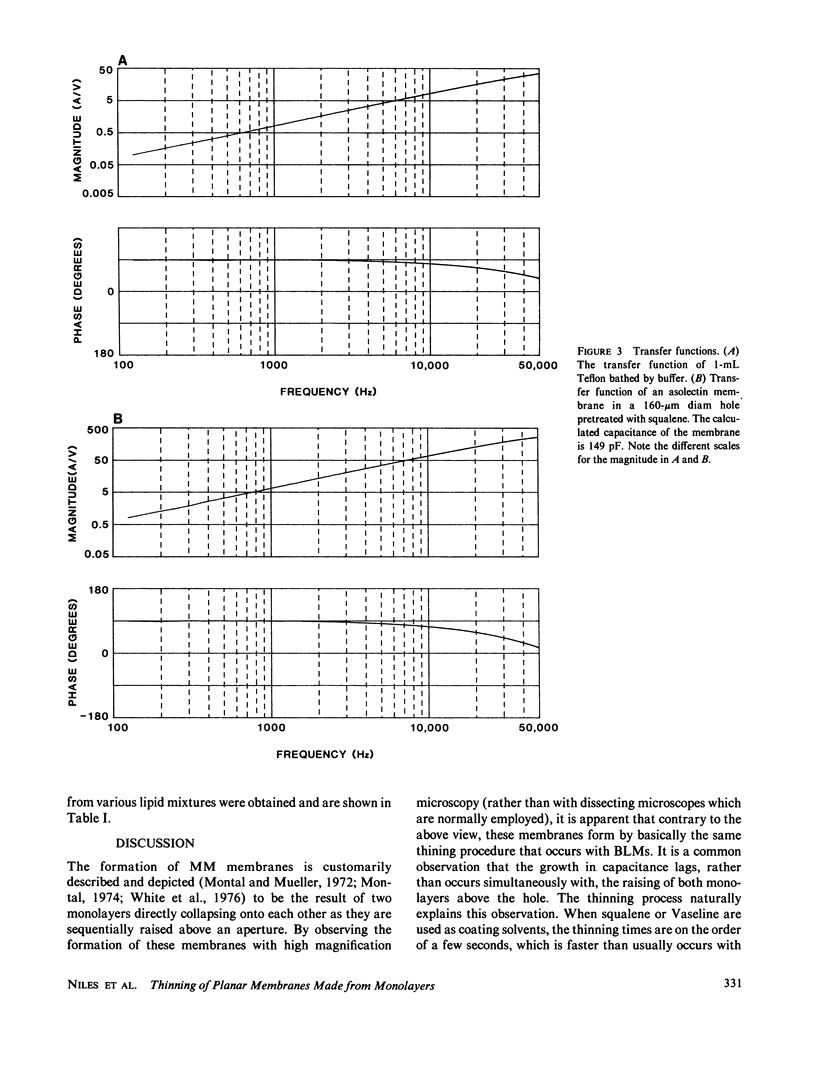
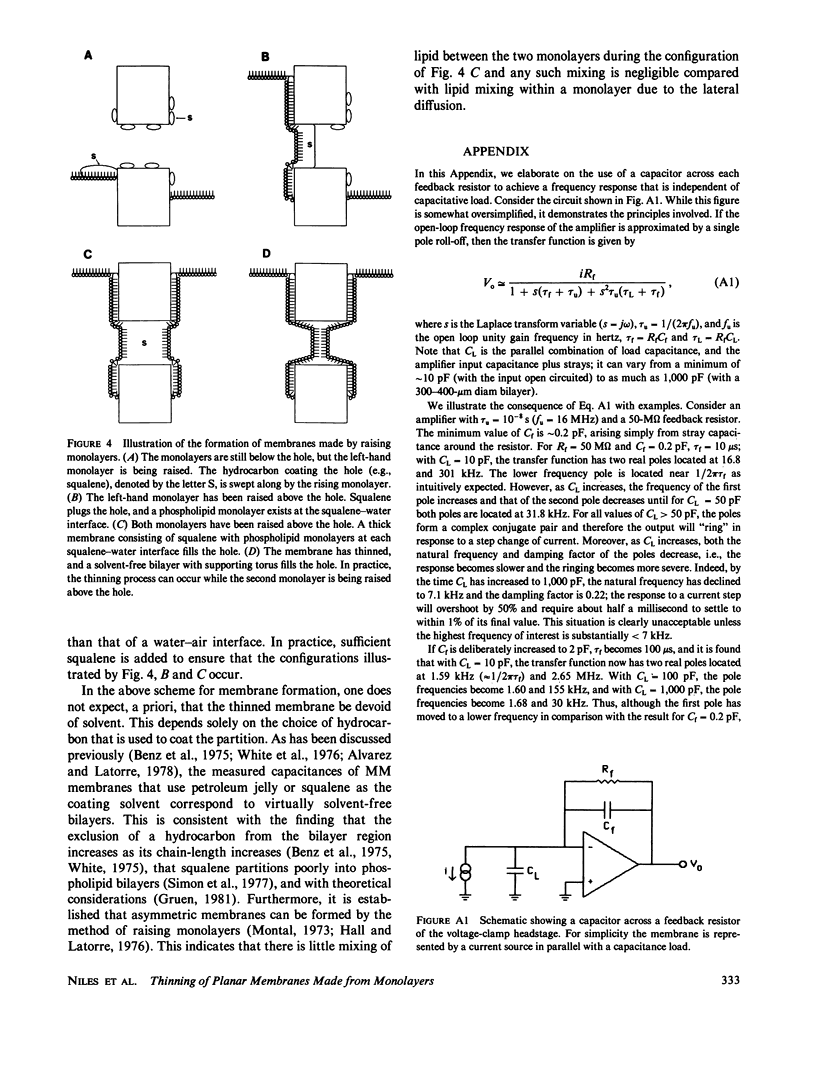
We investigated the manner in which planar phospholipid membranes form when monolayers are sequentially raised. Simultaneous electrical and optical recordings showed that initially a thick film forms, and the capacitance of the film increases with the same time course as the observed thinning. The diameter of fully thinned membranes varies from membrane to membrane and a torus is readily observed. The frequency-dependent admittance of the membrane was measured using a wide-bandwidth voltage clamp whose frequency response is essentially independent of capacitative load. The membrane capacitance dominates the total admittance and the membrane dielectric is not lossy. The specific capacitance of membranes of several mixtures was measured. A schematic diagram of the formation of these membranes is presented.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez O., Latorre R. Voltage-dependent capacitance in lipid bilayers made from monolayers. Biophys J. 1978 Jan;21(1):1–17. doi: 10.1016/S0006-3495(78)85505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Fröhlich O., Läuger P., Montal M. Electrical capacity of black lipid films and of lipid bilayers made from monolayers. Biochim Biophys Acta. 1975 Jul 3;394(3):323–334. doi: 10.1016/0005-2736(75)90287-4. [DOI] [PubMed] [Google Scholar]
- Gruen D. W. A mean-field model of the alkane-saturated lipid bilayer above its phase transition. I. Development of the model. Biophys J. 1981 Feb;33(2):149–166. doi: 10.1016/S0006-3495(81)84878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. E., Latorre R. Nonactin-K+ complex as a probe for membrane asymmetry. Biophys J. 1976 Jan;16(1):99–103. doi: 10.1016/S0006-3495(76)85667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal M. Asymmetric lipid bilayers. Reponse to multivalent ions. Biochim Biophys Acta. 1973 Mar 29;298(3):750–754. doi: 10.1016/0005-2736(73)90092-8. [DOI] [PubMed] [Google Scholar]
- Montal M. Formation of bimolecular membranes from lipid monolayers. Methods Enzymol. 1974;32:545–554. doi: 10.1016/0076-6879(74)32053-8. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles W. D., Cohen F. S. Video fluorescence microscopy studies of phospholipid vesicle fusion with a planar phospholipid membrane. Nature of membrane-membrane interactions and detection of release of contents. J Gen Physiol. 1987 Nov;90(5):703–735. doi: 10.1085/jgp.90.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes J., Latorre R. Effect of the anesthetics benzyl alcohol and chloroform on bilayers made from monolayers. Biophys J. 1979 Nov;28(2):259–279. doi: 10.1016/S0006-3495(79)85175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., Lis L. J., MacDonald R. C., Kauffman J. W. The noneffect of a large linear hydrocarbon, squalene, on the phosphatidylcholine packing structure. Biophys J. 1977 Jul;19(1):83–90. doi: 10.1016/S0006-3495(77)85570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Depth of water penetration into lipid bilayers. Methods Enzymol. 1986;127:511–521. doi: 10.1016/0076-6879(86)27041-x. [DOI] [PubMed] [Google Scholar]
- White S. H. Analysis of the torus surrounding planar lipid bilayer membranes. Biophys J. 1972 Apr;12(4):432–445. doi: 10.1016/S0006-3495(72)86095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H. Formation of "solvent-free" black lipid bilayer membranes from glyceryl monooleate dispersed in squalene. Biophys J. 1978 Sep;23(3):337–347. doi: 10.1016/S0006-3495(78)85453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H., Petersen D. C., Simon S., Yafuso M. Formation of planar bilayer membranes from lipid monolayers. A critique. Biophys J. 1976 May;16(5):481–489. doi: 10.1016/S0006-3495(76)85703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H. Phase transitions in planar bilayer membranes. Biophys J. 1975 Feb;15(2 Pt 1):95–117. doi: 10.1016/s0006-3495(75)85795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]