Abstract
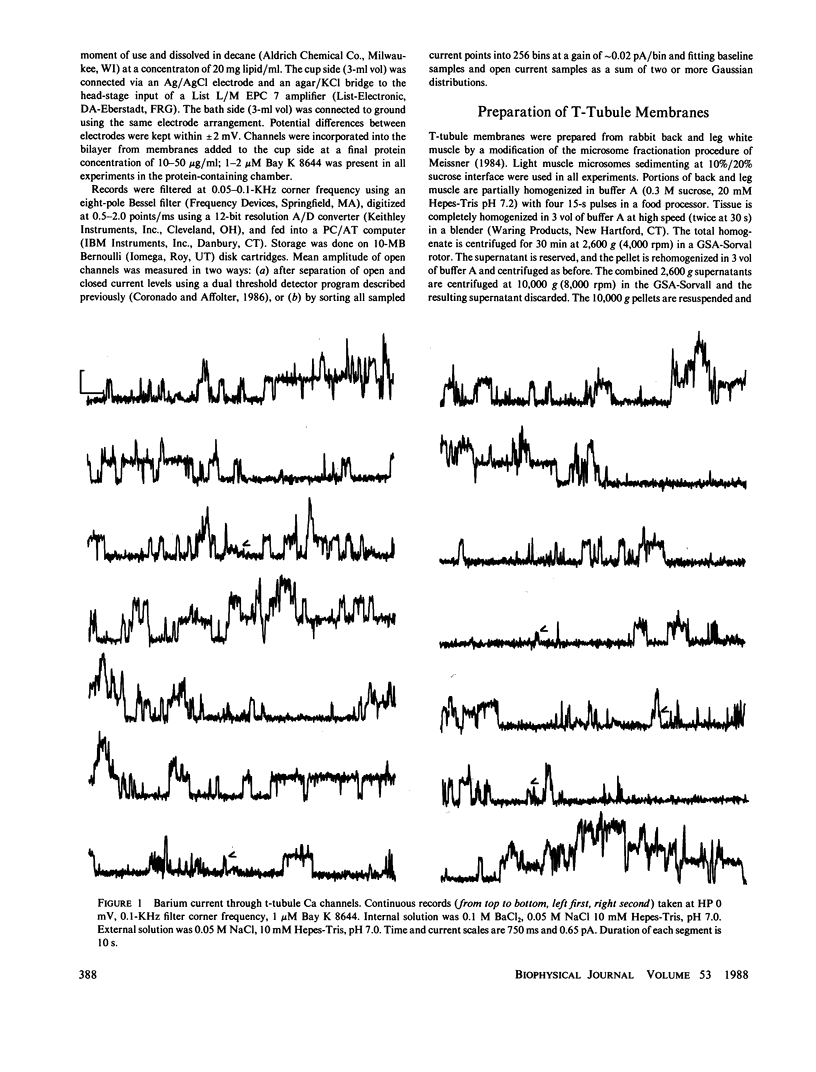
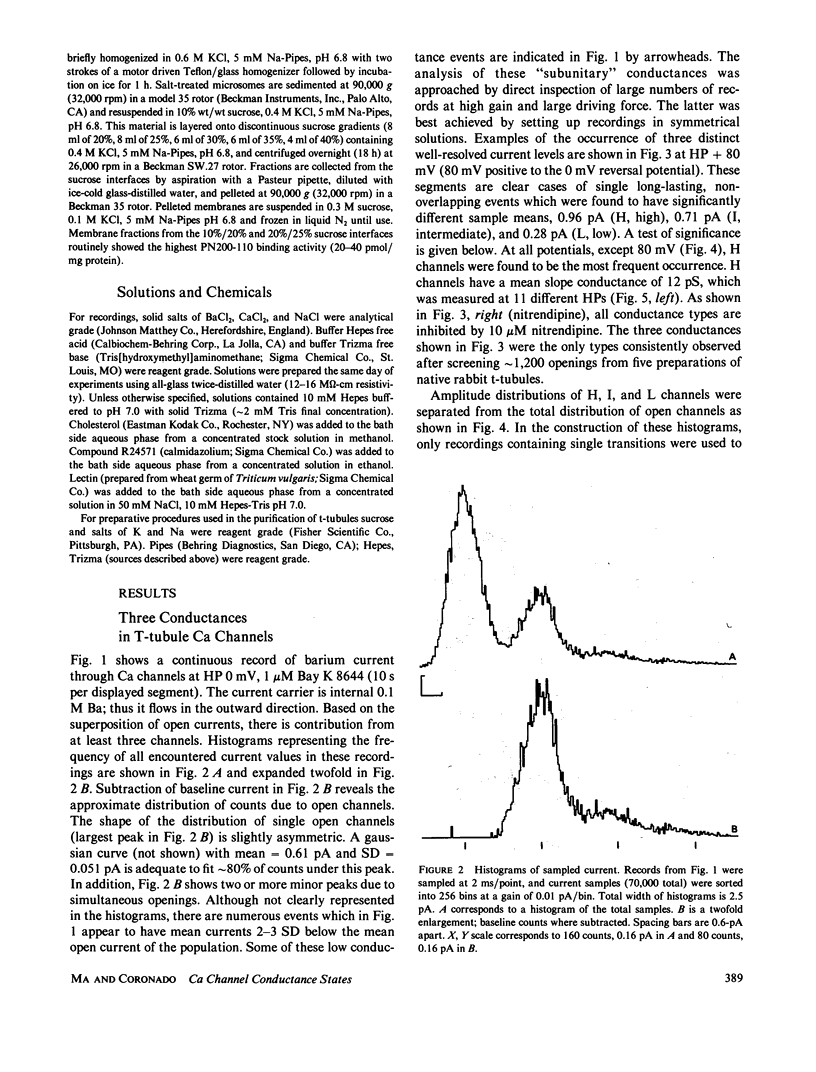
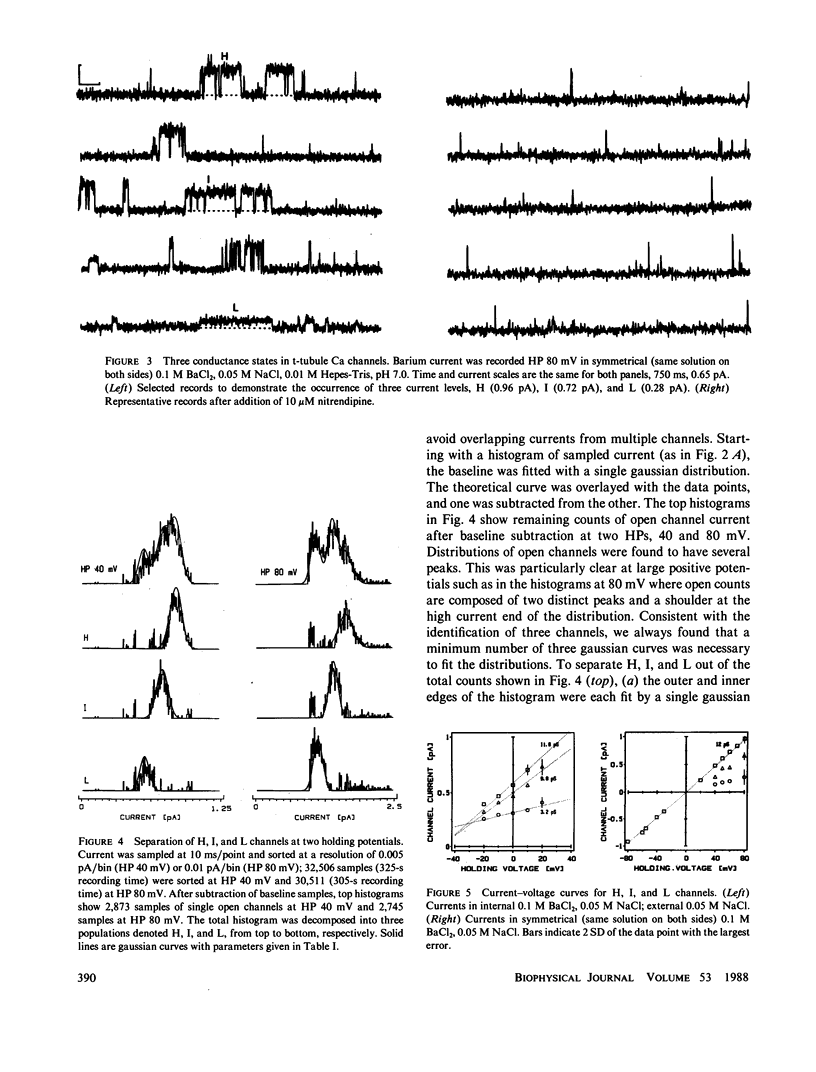
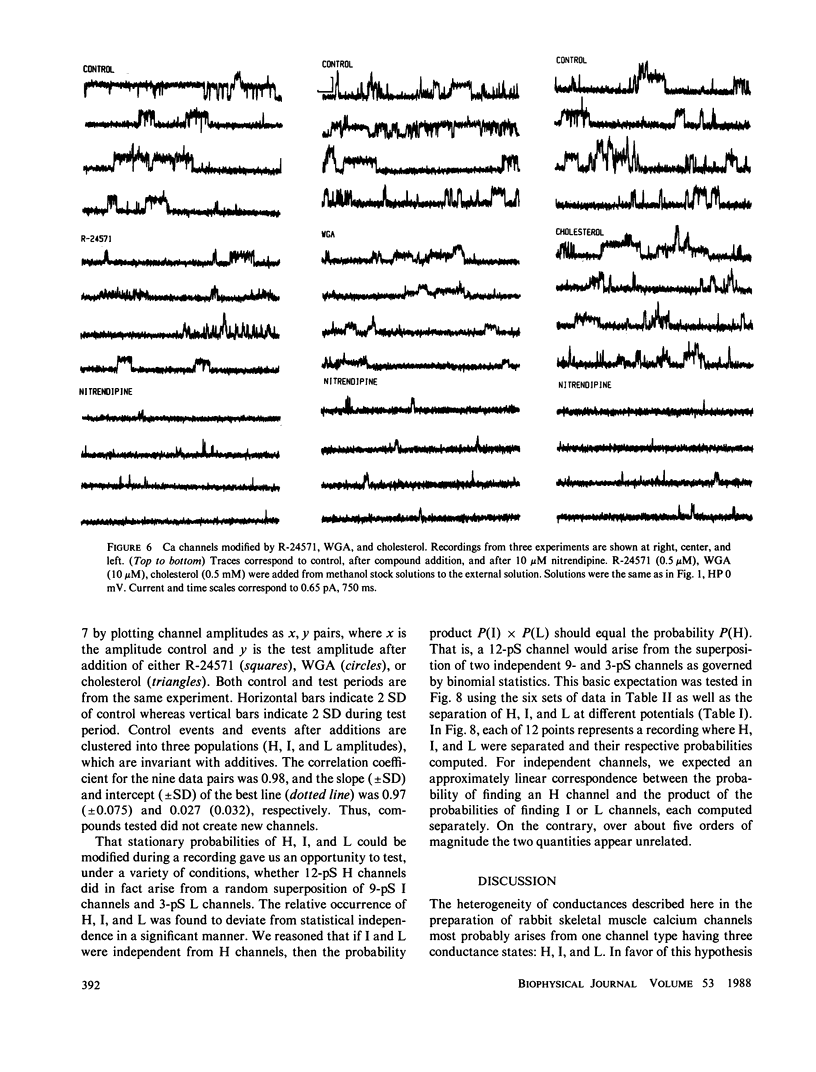
The single channel conductance of the dihydropyridine (DHP)-sensitive calcium channel from rabbit skeletal muscle transverse tubules was analyzed in detail using the planar bilayer recording technique. With 0.1 M BaCl2 on both sides of the channel (symmetrical solutions), the most frequent conductance is 12 pS, which is independent of holding potential in the range of -80 to +80 mV. This conductance accounts for approximately 80% of all openings analyzed close to 0 mV. Two additional channels of conductance 9 and 3 pS are also present at all positive potentials, but their relative occurrence close to 0 mV is low. All channels depend on the presence of agonist Bay K 8644 and are inhibited by the antagonist nitrendipine. The relative occurrence of 9 and 3 pS can be increased, and that of 12 pS decreased, by several interventions such as external addition of cholesterol, lectin (wheat germ agglutinin), or calmodulin inhibitor R24571 (calmidazolium). The 9- and 3-pS channels are also conspicuous at positive potentials larger than +40 mV. We suggest that 9- and 3-pS channels are two elementary conductances of the same DHP-sensitive Ca channel. Under most circumstances, these two conductances are gated in a coupled way to generate a channel with a unitary conductance of 12 pS. Interventions tested, including large depolarizations, probably decompose or uncouple the 12-pS channel into 9 and 3 pS.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter H., Coronado R. Agonists Bay-K8644 and CGP-28392 open calcium channels reconstituted from skeletal muscle transverse tubules. Biophys J. 1985 Aug;48(2):341–347. doi: 10.1016/S0006-3495(85)83789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Single low-voltage-activated calcium channels in chick and rat sensory neurones. J Physiol. 1987 May;386:571–601. doi: 10.1113/jphysiol.1987.sp016552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado R., Affolter H. Insulation of the conduction pathway of muscle transverse tubule calcium channels from the surface charge of bilayer phospholipid. J Gen Physiol. 1986 Jun;87(6):933–953. doi: 10.1085/jgp.87.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado R., Smith J. S. Monovalent ion current through single calcium channels of skeletal muscle transverse tubules. Biophys J. 1987 Mar;51(3):497–502. doi: 10.1016/S0006-3495(87)83371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Reconstitution of the voltage-sensitive calcium channel purified from skeletal muscle transverse tubules. Biochemistry. 1986 Jun 3;25(11):3077–3083. doi: 10.1021/bi00359a002. [DOI] [PubMed] [Google Scholar]
- Donaldson P. L., Beam K. G. Calcium currents in a fast-twitch skeletal muscle of the rat. J Gen Physiol. 1983 Oct;82(4):449–468. doi: 10.1085/jgp.82.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich B. E., Schen C. R., Garcia M. L., Kaczorowski G. J. Incorporation of calcium channels from cardiac sarcolemmal membrane vesicles into planar lipid bilayers. Proc Natl Acad Sci U S A. 1986 Jan;83(1):193–197. doi: 10.1073/pnas.83.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flockerzi V., Oeken H. J., Hofmann F., Pelzer D., Cavalié A., Trautwein W. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986 Sep 4;323(6083):66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- Galizzi J. P., Borsotto M., Barhanin J., Fosset M., Lazdunski M. Characterization and photoaffinity labeling of receptor sites for the Ca2+ channel inhibitors d-cis-diltiazem, (+/-)-bepridil, desmethoxyverapamil, and (+)-PN 200-110 in skeletal muscle transverse tubule membranes. J Biol Chem. 1986 Jan 25;261(3):1393–1397. [PubMed] [Google Scholar]
- Hanke W., Miller C. Single chloride channels from Torpedo electroplax. Activation by protons. J Gen Physiol. 1983 Jul;82(1):25–45. doi: 10.1085/jgp.82.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. D. A calmodulin-like ca receptor in the ca channel. Biophys J. 1984 Jan;45(1):134–136. doi: 10.1016/S0006-3495(84)84138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman J. B., Hess P., Tsien R. W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J Gen Physiol. 1986 Sep;88(3):321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. J Biol Chem. 1984 Feb 25;259(4):2365–2374. [PubMed] [Google Scholar]
- Nakayama N., Kirley T. L., Vaghy P. L., McKenna E., Schwartz A. Purification of putative Ca2+ channel protein from rabbit skeletal muscle. Determination of the amino-terminal sequence. J Biol Chem. 1987 May 15;262(14):6572–6576. [PubMed] [Google Scholar]
- Nelson M. T. Interactions of divalent cations with single calcium channels from rat brain synaptosomes. J Gen Physiol. 1986 Feb;87(2):201–222. doi: 10.1085/jgp.87.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature. 1987 Sep 17;329(6136):243–246. doi: 10.1038/329243a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. L., Hess P., Reeves J. P., Smilowitz H., Tsien R. W. Calcium channels in planar lipid bilayers: insights into mechanisms of ion permeation and gating. Science. 1986 Mar 28;231(4745):1564–1566. doi: 10.1126/science.2420007. [DOI] [PubMed] [Google Scholar]


