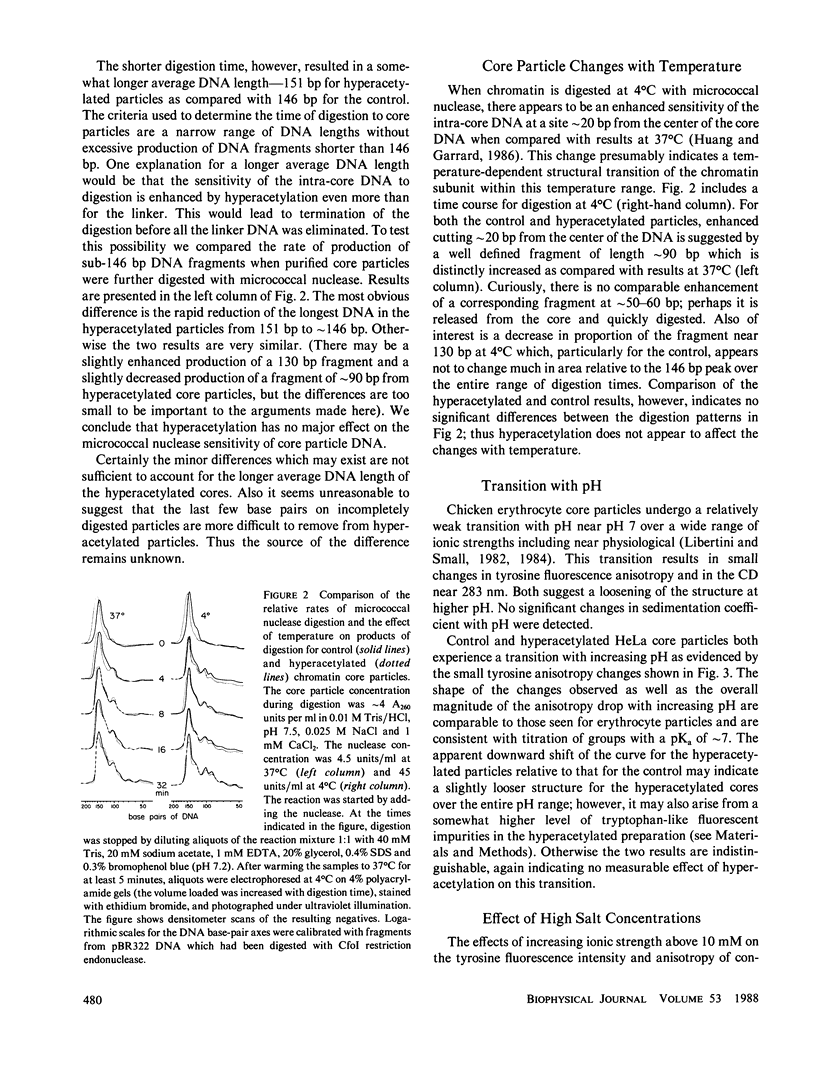
Abstract
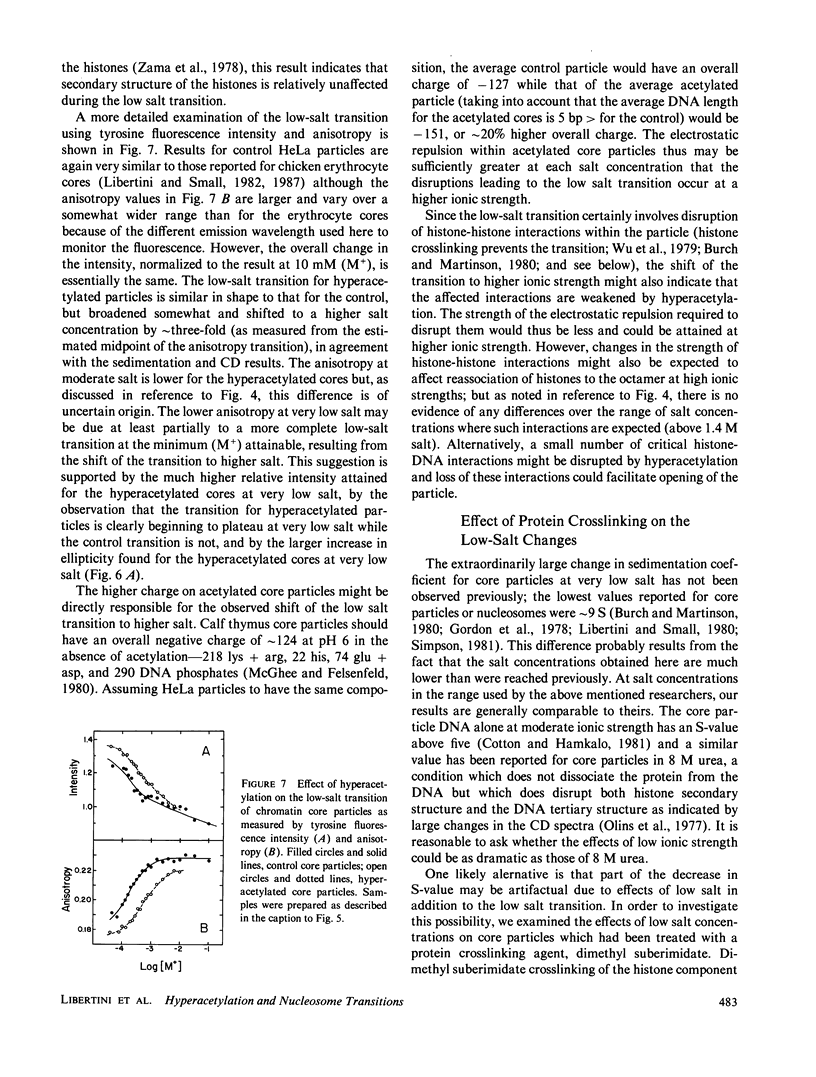
Effects of histone hyperacetylation on transitions of HeLa cell nucleosome core particles were studied. The transitions examined were induced by low salt concentrations, pH, temperature, and nondissociating high salt. Effects of salt dissociation were also examined. The low-salt transition was found to shift to higher ionic strength by approximately three fold for hyperacetylated particles, a change which may be due simply to the increased overall negative charge on the particles caused by acetylation of lysine residues. Some differences were also seen in the way in which core particles refold after exposure to very low salt (which induces a nonreversible change in the particles). Otherwise no significant effects of hyperacetylation were observed.
Full text
PDF


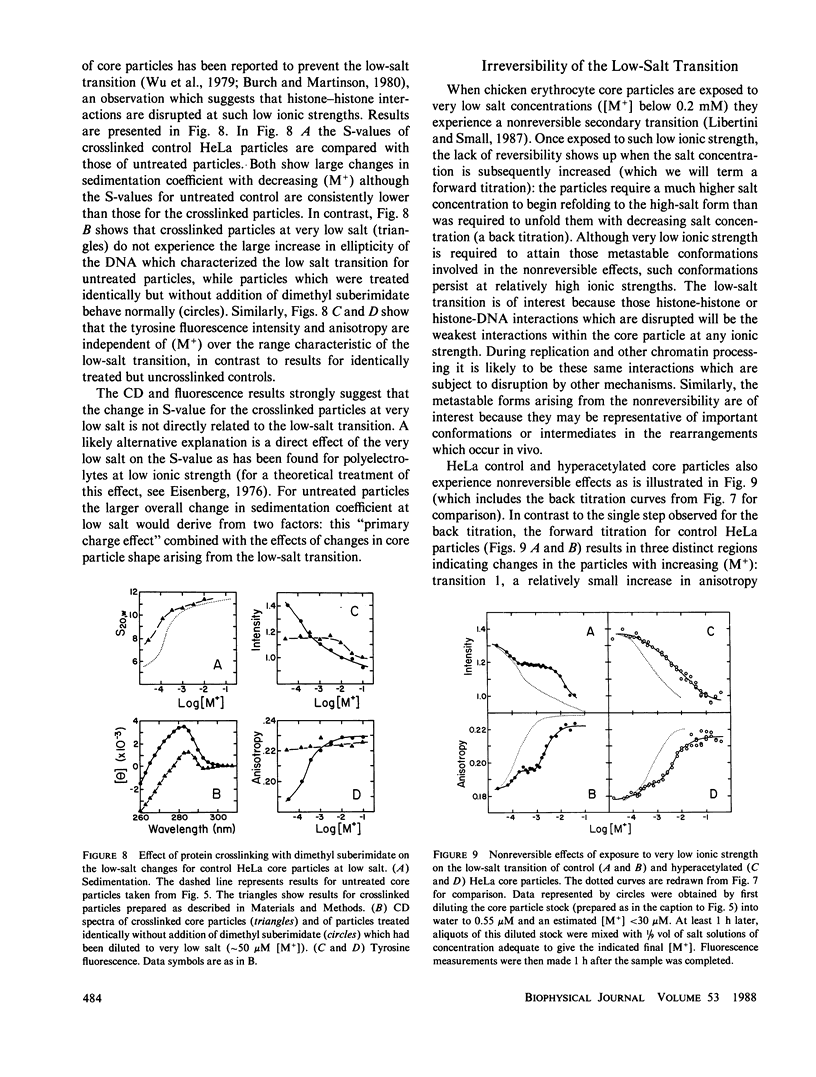


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausio J., van Holde K. E. Histone hyperacetylation: its effects on nucleosome conformation and stability. Biochemistry. 1986 Mar 25;25(6):1421–1428. doi: 10.1021/bi00354a035. [DOI] [PubMed] [Google Scholar]
- Ayres W. A., Small E. W., Isenberg I. A computerized fluorescence anisotropy spectrometer. Anal Biochem. 1974 Apr;58(2):361–367. doi: 10.1016/0003-2697(74)90203-6. [DOI] [PubMed] [Google Scholar]
- Bertrand E., Erard M., Gómez-Lira M., Bode J. Influence of histone hyperacetylation on nucleosomal particles as visualized by electron microscopy. Arch Biochem Biophys. 1984 Feb 15;229(1):395–398. doi: 10.1016/0003-9861(84)90167-x. [DOI] [PubMed] [Google Scholar]
- Bode J., Gómez-Lira M. M., Schröter H. Nucleosomal particles open as the histone core becomes hyperacetylated. Eur J Biochem. 1983 Feb 15;130(3):437–445. doi: 10.1111/j.1432-1033.1983.tb07170.x. [DOI] [PubMed] [Google Scholar]
- Bode J., Henco K., Wingender E. Modulation of the nucleosome structure by histone acetylation. Eur J Biochem. 1980 Sep;110(1):143–152. doi: 10.1111/j.1432-1033.1980.tb04849.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., West M. H., Stedman J. D. Two-dimensional gel analysis of histones in acid extracts of nuclei, cells, and tissues. Eur J Biochem. 1980 Aug;109(1):17–23. doi: 10.1111/j.1432-1033.1980.tb04762.x. [DOI] [PubMed] [Google Scholar]
- Burch J. B., Martinson H. G. The roles of H1, the histone core and DNA length in the unfolding of nucleosomes at low ionic strength. Nucleic Acids Res. 1980 Nov 11;8(21):4969–4987. doi: 10.1093/nar/8.21.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P. J. A defined structure of the 30 nm chromatin fibre which accommodates different nucleosomal repeat lengths. EMBO J. 1984 Nov;3(11):2599–2604. doi: 10.1002/j.1460-2075.1984.tb02180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R. W., Hamkalo B. A. Nucleosome dissociation at physiological ionic strengths. Nucleic Acids Res. 1981 Jan 24;9(2):445–457. doi: 10.1093/nar/9.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman M. K., Fasman G. D. Circular dichroism analysis of mononucleosome DNA conformation. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4759–4763. doi: 10.1073/pnas.75.10.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
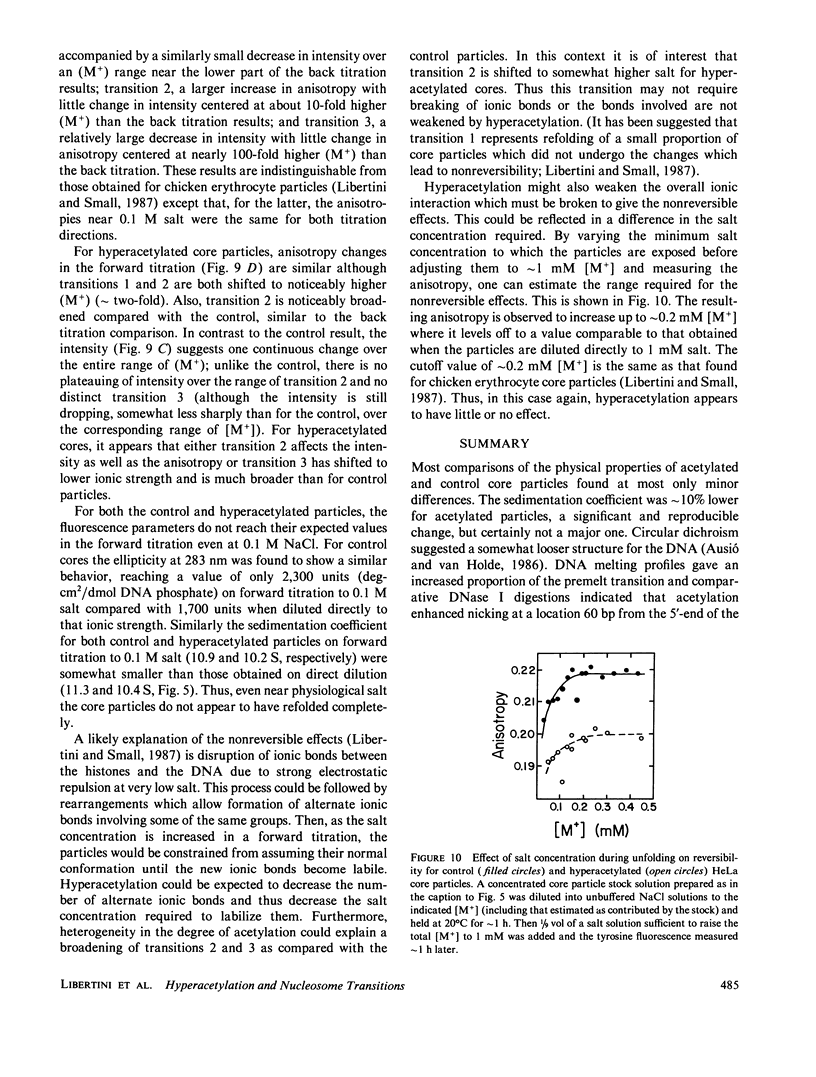
- Doenecke D., Gallwitz D. Acetylation of histones in nucleosomes. Mol Cell Biochem. 1982 Apr 30;44(2):113–128. doi: 10.1007/BF00226895. [DOI] [PubMed] [Google Scholar]
- Eickbush T. H., Moudrianakis E. N. The histone core complex: an octamer assembled by two sets of protein-protein interactions. Biochemistry. 1978 Nov 14;17(23):4955–4964. doi: 10.1021/bi00616a016. [DOI] [PubMed] [Google Scholar]
- Eisenberg H. Sedimentation in the ultracentrifuge and diffusion of macromolecules carrying electrical charges. Biophys Chem. 1976 Jul;5(1-2):243–251. doi: 10.1016/0301-4622(76)80037-3. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Bellard M., Oudet P., Chambon P. Stability of nucleosomes in native and reconstituted chromatins. Nucleic Acids Res. 1976 Nov;3(11):3173–3192. doi: 10.1093/nar/3.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey J. E., Eickbush T. H., Moudrianakis E. N. Reversible association of calf thymus histones to form the symmetrical octamer (H2AH2BH3H4)2: a case of a mixed-associating system. Biochemistry. 1980 Apr 1;19(7):1339–1346. doi: 10.1021/bi00548a012. [DOI] [PubMed] [Google Scholar]
- Gordon V. C., Knobler C. M., Olins D. E., Schumaker V. N. Conformational changes of the chromatin subunit. Proc Natl Acad Sci U S A. 1978 Feb;75(2):660–663. doi: 10.1073/pnas.75.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. Y., Garrard W. T. Temperature-dependent cleavage of chromatin by micrococcal nuclease near the nucleosome center. FEBS Lett. 1986 Apr 7;199(1):89–91. doi: 10.1016/0014-5793(86)81229-7. [DOI] [PubMed] [Google Scholar]
- Imai B. S., Yau P., Baldwin J. P., Ibel K., May R. P., Bradbury E. M. Hyperacetylation of core histones does not cause unfolding of nucleosomes. Neutron scatter data accords with disc shape of the nucleosome. J Biol Chem. 1986 Jul 5;261(19):8784–8792. [PubMed] [Google Scholar]
- Isenberg I. Histones. Annu Rev Biochem. 1979;48:159–191. doi: 10.1146/annurev.bi.48.070179.001111. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Libertini L. J., Small E. W. Effects of pH on low-salt transition of chromatin core particles. Biochemistry. 1982 Jul 6;21(14):3327–3334. doi: 10.1021/bi00257a013. [DOI] [PubMed] [Google Scholar]
- Libertini L. J., Small E. W. Effects of pH on the stability of chromatin core particles. Nucleic Acids Res. 1984 May 25;12(10):4351–4359. doi: 10.1093/nar/12.10.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertini L. J., Small E. W. Reversibility of the low-salt transition of chromatin core particles. Nucleic Acids Res. 1987 Aug 25;15(16):6655–6664. doi: 10.1093/nar/15.16.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertini L. J., Small E. W. Salt induced transitions of chromatin core particles studied by tyrosine fluorescence anisotropy. Nucleic Acids Res. 1980 Aug 25;8(16):3517–3534. doi: 10.1093/nar/8.16.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertini L. J., Small E. W. The intrinsic tyrosine fluorescence of histone H1. Steady state and fluorescence decay studies reveal heterogeneous emission. Biophys J. 1985 Jun;47(6):765–772. doi: 10.1016/S0006-3495(85)83979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Dityrosine formation in calmodulin. Biochemistry. 1987 Feb 10;26(3):695–704. doi: 10.1021/bi00377a006. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Bryan P. N., Harrington R. E., Hill W. E., Olins A. L. Conformational states of chromatin nu bodies induced by urea. Nucleic Acids Res. 1977 Jun;4(6):1911–1931. doi: 10.1093/nar/4.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M., Chalkley R. The effect of histone hyperacetylation on the nuclease sensitivity and the solubility of chromatin. J Biol Chem. 1981 Apr 10;256(7):3313–3318. [PubMed] [Google Scholar]
- Reeves R. Transcriptionally active chromatin. Biochim Biophys Acta. 1984 Sep 10;782(4):343–393. doi: 10.1016/0167-4781(84)90044-7. [DOI] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger F. B., Dattagupta N., Crothers D. M. Unfolding of 175-base-pair nucleosomes. Biochemistry. 1982 Feb 16;21(4):664–669. doi: 10.1021/bi00533a012. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Mechanism of a reversible, thermally induced conformational change in chromatin core particles. J Biol Chem. 1979 Oct 25;254(20):10123–10127. [PubMed] [Google Scholar]
- Simpson R. T. Modulation of nucleosome structure by histone subtypes in sea urchin embryos. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6803–6807. doi: 10.1073/pnas.78.11.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T. Structure of chromatin containing extensively acetylated H3 and H4. Cell. 1978 Apr;13(4):691–699. doi: 10.1016/0092-8674(78)90219-2. [DOI] [PubMed] [Google Scholar]
- Weischet W. O., Tatchell K., Van Holde K. E., Klump H. Thermal denaturation of nucleosomal core particles. Nucleic Acids Res. 1978 Jan;5(1):139–160. doi: 10.1093/nar/5.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Dattagupta N., Hogan M., Crothers D. M. Structural changes of nucleosomes in low-salt concentrations. Biochemistry. 1979 Sep 4;18(18):3960–3965. doi: 10.1021/bi00585a018. [DOI] [PubMed] [Google Scholar]
- Yager T. D., van Holde K. E. Dynamics and equilibria of nucleosomes at elevated ionic strength. J Biol Chem. 1984 Apr 10;259(7):4212–4222. [PubMed] [Google Scholar]
- Zama M., Olins D. E., Prescott B., Thomas G. J., Jr Nucleosome conformation: pH and organic solvent effects. Nucleic Acids Res. 1978 Oct;5(10):3881–3897. doi: 10.1093/nar/5.10.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]