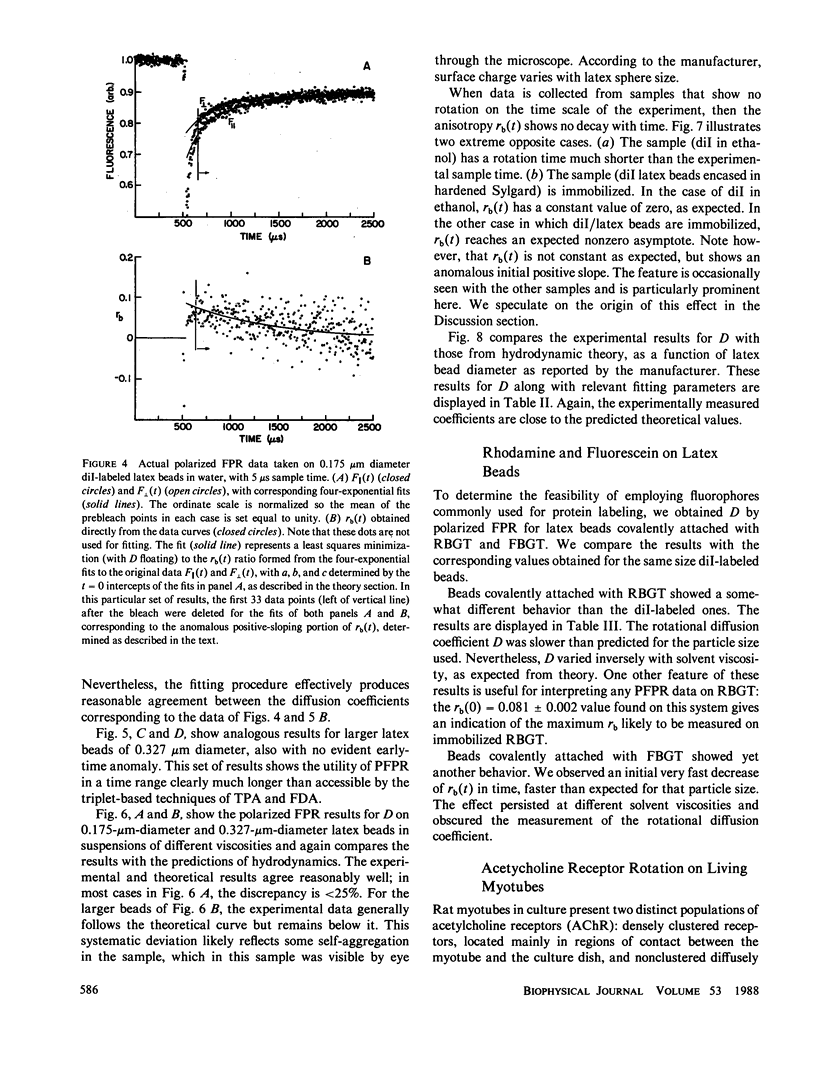
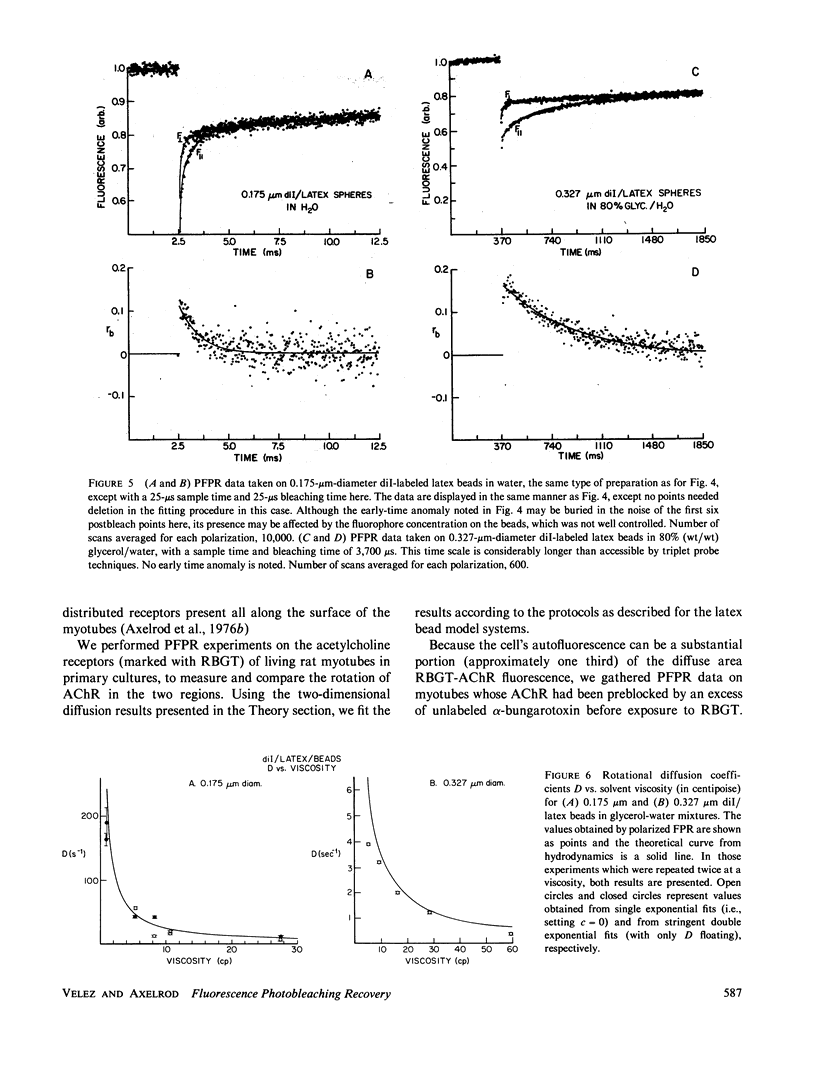
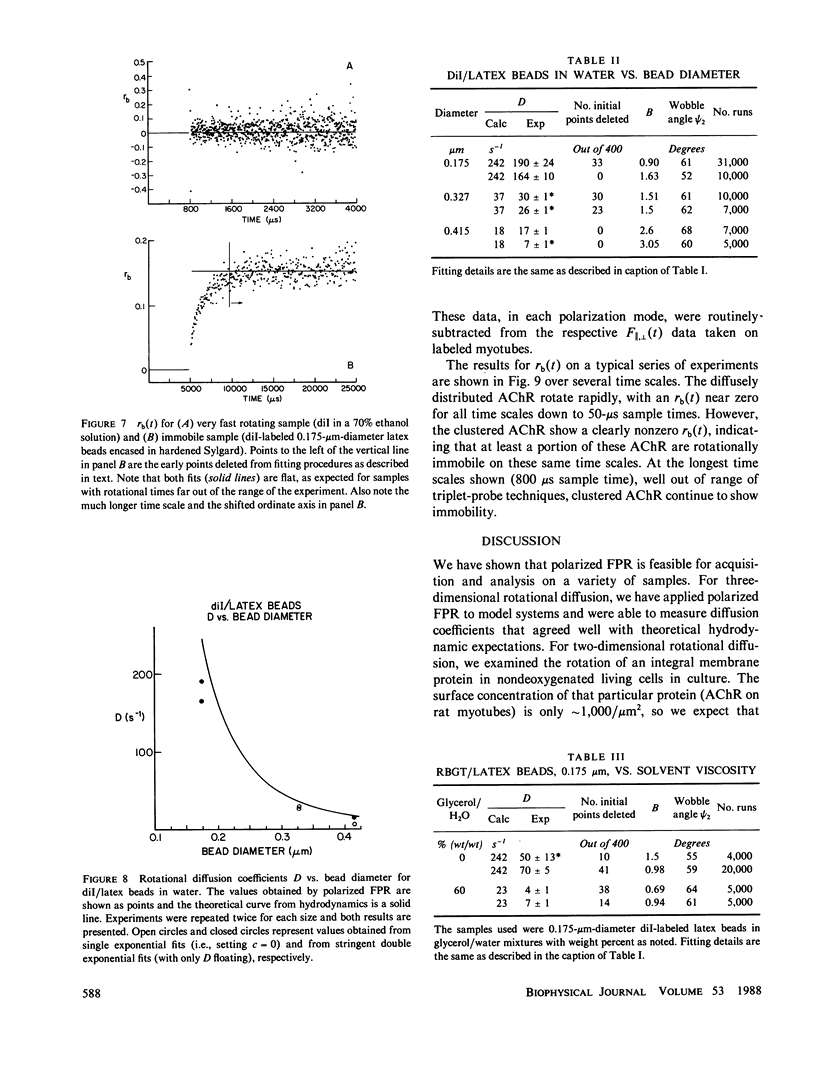
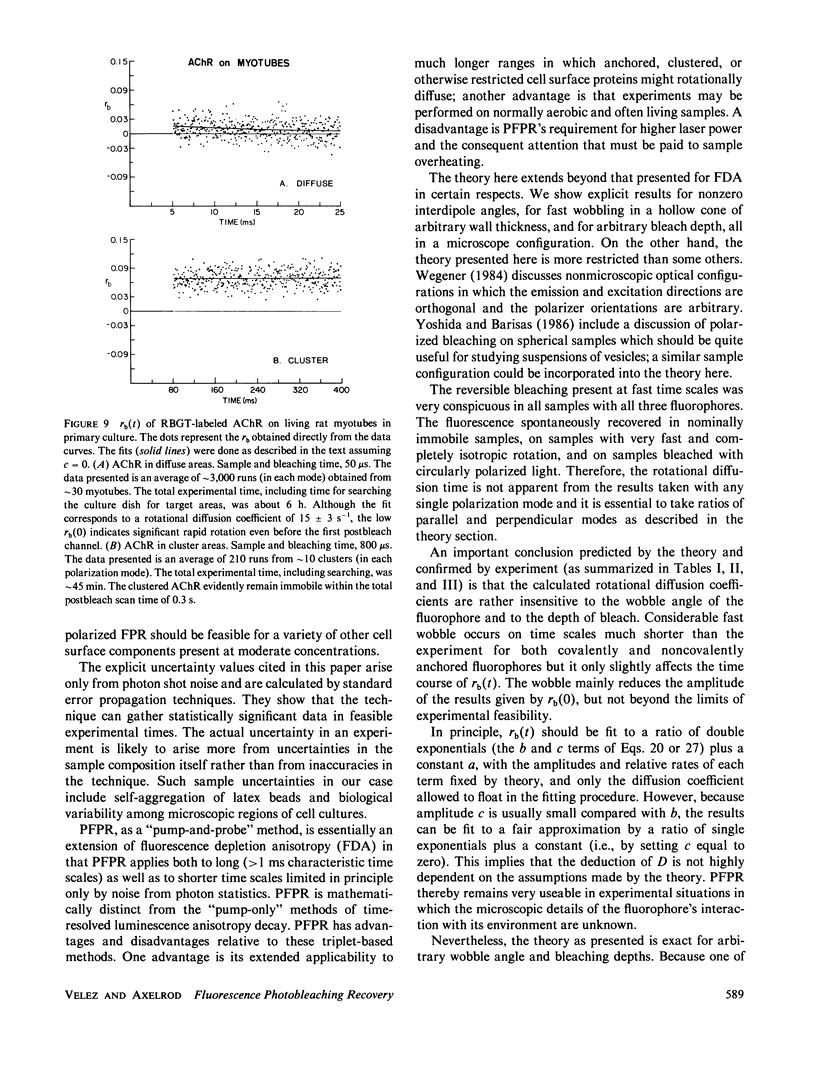
Abstract
A variation of fluorescence photobleaching recovery (FPR) suitable for measuring the rate of rotational molecular diffusion in solution and cell membranes is presented in theory and experimental practice for epi-illumination microscopy. In this technique, a brief flash of polarized laser light creates an anisotropic distribution of unbleached fluorophores which relaxes by rotational diffusion, leading to a time-dependent postbleach fluorescence. Polarized FPR (PFPR) is applicable to any time scales from seconds to microseconds. However, at fast (microsecond) time scales, a partial recovery independent of molecular orientation tends to obscure rotational effects. The theory here presents a method for overcoming this reversible photobleaching, and includes explicit results for practical geometries, fast wobble of fluorophores, and arbitrary bleaching depth. This variation of a polarized luminescence "pump-and-probe" technique is compared with prior ones and with "pump-only" time-resolved luminescence anisotropy decay methods. The technique is experimentally verified on small latex beads with a variety of diameters, common fluorophore labels, and solvent viscosities. Preliminary measurements on a protein (acetylcholine receptor) in the membrane of nondeoxygenated cells in live culture (rat myotubes) show a difference in rotational diffusion between clustered and nonclustered receptors. In most experiments, signal averaging, high laser power, and automated sample translation must be employed to achieve adequate statistical accuracy.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D. Carbocyanine dye orientation in red cell membrane studied by microscopic fluorescence polarization. Biophys J. 1979 Jun;26(3):557–573. doi: 10.1016/S0006-3495(79)85271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. Cell surface heating during fluorescence photobleaching recovery experiments. Biophys J. 1977 Apr;18(1):129–131. doi: 10.1016/S0006-3495(77)85601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. Lateral motion of membrane proteins and biological function. J Membr Biol. 1983;75(1):1–10. doi: 10.1007/BF01870794. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P., Koppel D. E., Schlessinger J., Webb W. W., Elson E. L., Podleski T. R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4594–4598. doi: 10.1073/pnas.73.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanovich S., Trón L., Szöllösi J., Zidovetzki R., Vaz W. L., Regateiro F., Arndt-Jovin D. J., Jovin T. M. Distribution and mobility of murine histocompatibility H-2Kk antigen in the cytoplasmic membrane. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5985–5989. doi: 10.1073/pnas.80.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch S. A., Piper H. M., Spieckermann P. G., Stier A. Anoxia influences the lateral diffusion of a lipid probe in the plasma membrane of isolated cardiac myocytes. Basic Res Cardiol. 1985;80 (Suppl 1):149–152. doi: 10.1007/978-3-662-11041-6_29. [DOI] [PubMed] [Google Scholar]
- Johnson P., Garland P. B. Depolarization of fluorescence depletion. A microscopic method for measuring rotational diffusion of membrane proteins on the surface of a single cell. FEBS Lett. 1981 Sep 28;132(2):252–256. doi: 10.1016/0014-5793(81)81172-6. [DOI] [PubMed] [Google Scholar]
- Johnson P., Garland P. B. Fluorescent triplet probes for measuring the rotational diffusion of membrane proteins. Biochem J. 1982 Apr 1;203(1):313–321. doi: 10.1042/bj2030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kawato S., Ikegami A. A theory of fluorescence polarization decay in membranes. Biophys J. 1977 Dec;20(3):289–305. doi: 10.1016/S0006-3495(77)85550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kawato S., Ikegami A. Dynamic structure of biological and model membranes: analysis by optical anisotropy decay measurement. Adv Biophys. 1984;17:147–203. doi: 10.1016/0065-227x(84)90027-3. [DOI] [PubMed] [Google Scholar]
- Lo M. M., Garland P. B., Lamprecht J., Barnard E. A. Rotational mobility of the membrane-bound acetylcholine receptor of Torpedo electric organ measured by phosphorescence depolarisation. FEBS Lett. 1980 Mar 10;111(2):407–412. doi: 10.1016/0014-5793(80)80838-6. [DOI] [PubMed] [Google Scholar]
- Scalettar B. A., Selvin P. R., Axelrod D., Hearst J. E., Klein M. P. A fluorescence photobleaching study of the microsecond reorientational motions of DNA. Biophys J. 1988 Feb;53(2):215–226. doi: 10.1016/S0006-3495(88)83083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Weis R. M., McConnell H. M. Measurement of rotational motion in membranes using fluorescence recovery after photobleaching. Biophys J. 1981 Oct;36(1):73–91. doi: 10.1016/S0006-3495(81)84717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener W. A. Fluorescence recovery spectroscopy as a probe of slow rotational motions. Biophys J. 1984 Dec;46(6):795–803. doi: 10.1016/S0006-3495(84)84078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener W. A., Rigler R. Separation of translational and rotational contributions in solution studies using fluorescence photobleaching recovery. Biophys J. 1984 Dec;46(6):787–793. doi: 10.1016/S0006-3495(84)84077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T. M., Barisas B. G. Protein rotational motion in solution measured by polarized fluorescence depletion. Biophys J. 1986 Jul;50(1):41–53. doi: 10.1016/S0006-3495(86)83437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


