Abstract
The effect of alamethicin and its derivatives on the voltage-dependent capacitance of phosphatidylethanolamine (squalane) membranes was measured using two different methods: lock-in detection and voltage pulse. Alamethicin and its derivatives modulate the voltage-dependent capacitance at voltages lower than the voltage at which alamethicin-induced conductance is detected. The magnitude and sign of this alamethicin-induced capacitance change depends on the aqueous alamethicin concentration and the kind of alamethicin used. Our experimental data can be interpreted as a potential-dependent pseudocapacitance associated with adsorbed alamethicin. Pseudocapacitance is expressed as a function of alamethicin charge, its concentration in the bathing solution and the applied electric field. The theory describes the dependence of the capacitance on applied voltage and alamethicin concentration. When alamethicin is neutral the theory predicts no change of the voltage-dependent capacitance with either sign of applied voltage. Experimental data are consistent with the model in which alamethicin molecules interact with each other while being adsorbed to the membrane surface. The energy of this interaction depends on the alamethicin concentration.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez O., Latorre R. Voltage-dependent capacitance in lipid bilayers made from monolayers. Biophys J. 1978 Jan;21(1):1–17. doi: 10.1016/S0006-3495(78)85505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee U., Tsui F. P., Balasubramanian T. N., Marshall G. R., Chan S. I. Structure of Alamethicin in solution. One- and two-dimensional 1H nuclear magnetic resonance studies at 500 MHz. J Mol Biol. 1983 Apr 25;165(4):757–775. doi: 10.1016/s0022-2836(83)80279-4. [DOI] [PubMed] [Google Scholar]
- Benz R., Fröhlich O., Läuger P., Montal M. Electrical capacity of black lipid films and of lipid bilayers made from monolayers. Biochim Biophys Acta. 1975 Jul 3;394(3):323–334. doi: 10.1016/0005-2736(75)90287-4. [DOI] [PubMed] [Google Scholar]
- Boheim G. Statistical analysis of alamethicin channels in black lipid membranes. J Membr Biol. 1974;19(3):277–303. doi: 10.1007/BF01869983. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Hall J. Alamethicin channels incorporated into frog node of ranvier: calcium-induced inactivation and membrane surface charges. J Gen Physiol. 1982 Mar;79(3):411–436. doi: 10.1085/jgp.79.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M., Hall J. E., Mead C. A. The nature of the voltage-dependent conductance induced by alamethicin in black lipid membranes. J Membr Biol. 1973 Dec 31;14(2):143–176. doi: 10.1007/BF01868075. [DOI] [PubMed] [Google Scholar]
- Fox R. O., Jr, Richards F. M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution. Nature. 1982 Nov 25;300(5890):325–330. doi: 10.1038/300325a0. [DOI] [PubMed] [Google Scholar]
- Fringeli U. P. Distribution and diffusion of alamethicin in a lecithin/water model membrane system. J Membr Biol. 1980 Jun 15;54(3):203–212. doi: 10.1007/BF01870236. [DOI] [PubMed] [Google Scholar]
- Gisin B. F., Kobayashi S., Hall J. E. Synthesis of a 19-residue peptide with alamethicin-like activity. Proc Natl Acad Sci U S A. 1977 Jan;74(1):115–119. doi: 10.1073/pnas.74.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon L. G., Haydon D. A. Potential-dependent conductances in lipid membranes containing alamethicin. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):433–447. doi: 10.1098/rstb.1975.0021. [DOI] [PubMed] [Google Scholar]
- Gordon L. G., Haydon D. A. The unit conductance channel of alamethicin. Biochim Biophys Acta. 1972 Mar 17;255(3):1014–1018. doi: 10.1016/0005-2736(72)90415-4. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Cahalan M. D. Calcium-induced inactivation of alamethicin in asymmetric lipid bilayers. J Gen Physiol. 1982 Mar;79(3):387–409. doi: 10.1085/jgp.79.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. E., Vodyanoy I., Balasubramanian T. M., Marshall G. R. Alamethicin. A rich model for channel behavior. Biophys J. 1984 Jan;45(1):233–247. doi: 10.1016/S0006-3495(84)84151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G., Dubischar N. Conformational changes of alamethicin induced by solvent and temperature. A 13C-NMR and circular-dichroism study. Eur J Biochem. 1975 Jun;54(2):395–409. doi: 10.1111/j.1432-1033.1975.tb04150.x. [DOI] [PubMed] [Google Scholar]
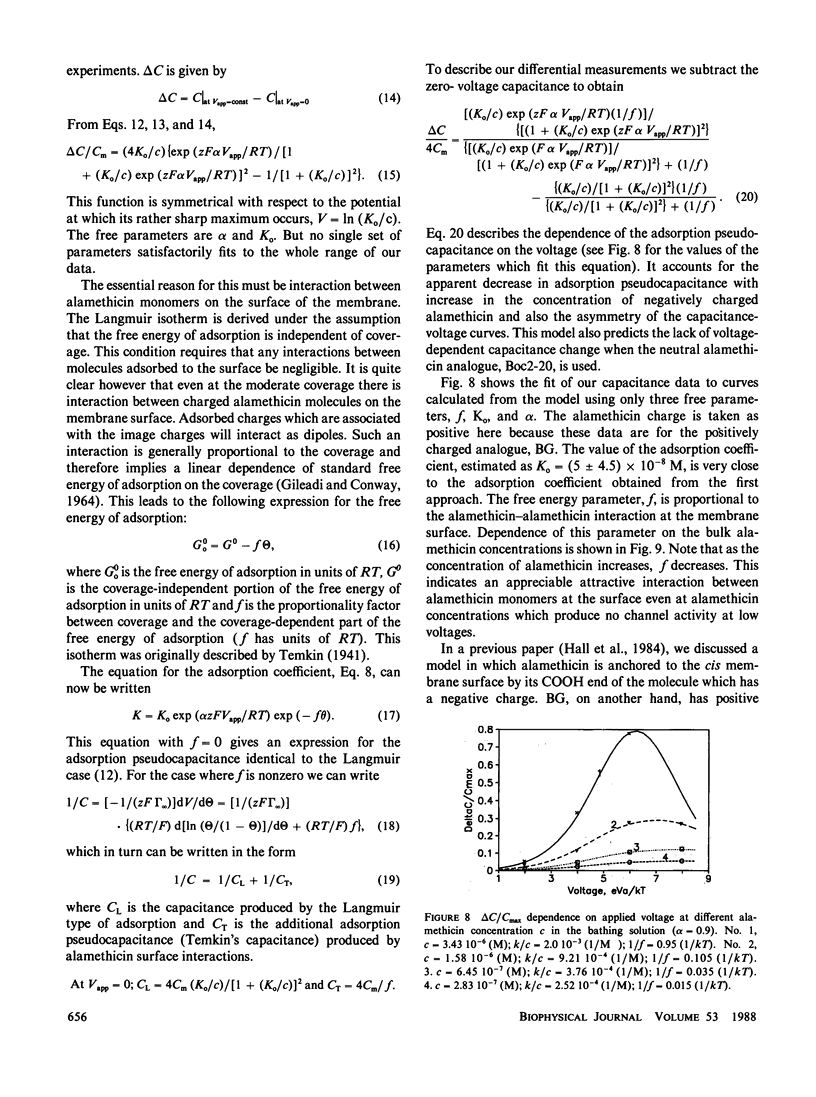
- Letter: Lenses and the compression of black lipid membranes by an electric field. Biophys J. 1975 Jan;15(1):77–81. doi: 10.1016/S0006-3495(75)85793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis L. J., Kauffman J. W., Shriver D. F. Raman spectroscopic detection and examination of the interaction of amino acids, polypeptides and proteins with the phophatidylcholine lamellar structure. Biochim Biophys Acta. 1976 Jul 1;436(3):513–522. doi: 10.1016/0005-2736(76)90437-5. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Action potentials induced in biomolecular lipid membranes. Nature. 1968 Feb 24;217(5130):713–719. doi: 10.1038/217713a0. [DOI] [PubMed] [Google Scholar]
- Reusser F. Biosynthesis of antibiotic U-22,324, a cyclic polypeptide. J Biol Chem. 1967 Jan 25;242(2):243–247. [PubMed] [Google Scholar]
- Rizzo V., Stankowski S., Schwarz G. Alamethicin incorporation in lipid bilayers: a thermodynamic study. Biochemistry. 1987 May 19;26(10):2751–2759. doi: 10.1021/bi00384a015. [DOI] [PubMed] [Google Scholar]
- Sargent D. F. Voltage jump/capacitance relaxation studies of bilayer structure and dynamics. Studies on oxidized cholesterol membranes. J Membr Biol. 1975;23(3-4):277–247. [PubMed] [Google Scholar]
- Schoch P., Sargent D. F., Schwyzer R. Capacitance and conductance as tools for the measurement of asymmetric surface potentials and energy barriers of lipid bilayer membranes. J Membr Biol. 1979 Apr 12;46(1):71–89. doi: 10.1007/BF01959975. [DOI] [PubMed] [Google Scholar]
- Schwarz G., Savko P. Structural and dipolar properties of the voltage-dependent pore former alamethicin in octanol/dioxane. Biophys J. 1982 Aug;39(2):211–219. doi: 10.1016/S0006-3495(82)84510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G., Stankowski S., Rizzo V. Thermodynamic analysis of incorporation and aggregation in a membrane: application to the pore-forming peptide alamethicin. Biochim Biophys Acta. 1986 Sep 25;861(1):141–151. doi: 10.1016/0005-2736(86)90573-0. [DOI] [PubMed] [Google Scholar]
- Vodyanoy I., Hall J. E., Balasubramanian T. M. Alamethicin-induced current-voltage curve asymmetry in lipid bilayers. Biophys J. 1983 Apr;42(1):71–82. doi: 10.1016/S0006-3495(83)84370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H., Chang W. Voltage dependence of the capacitance and area of black lipid membranes. Biophys J. 1981 Nov;36(2):449–453. doi: 10.1016/S0006-3495(81)84744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H. Formation of "solvent-free" black lipid bilayer membranes from glyceryl monooleate dispersed in squalene. Biophys J. 1978 Sep;23(3):337–347. doi: 10.1016/S0006-3495(78)85453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


