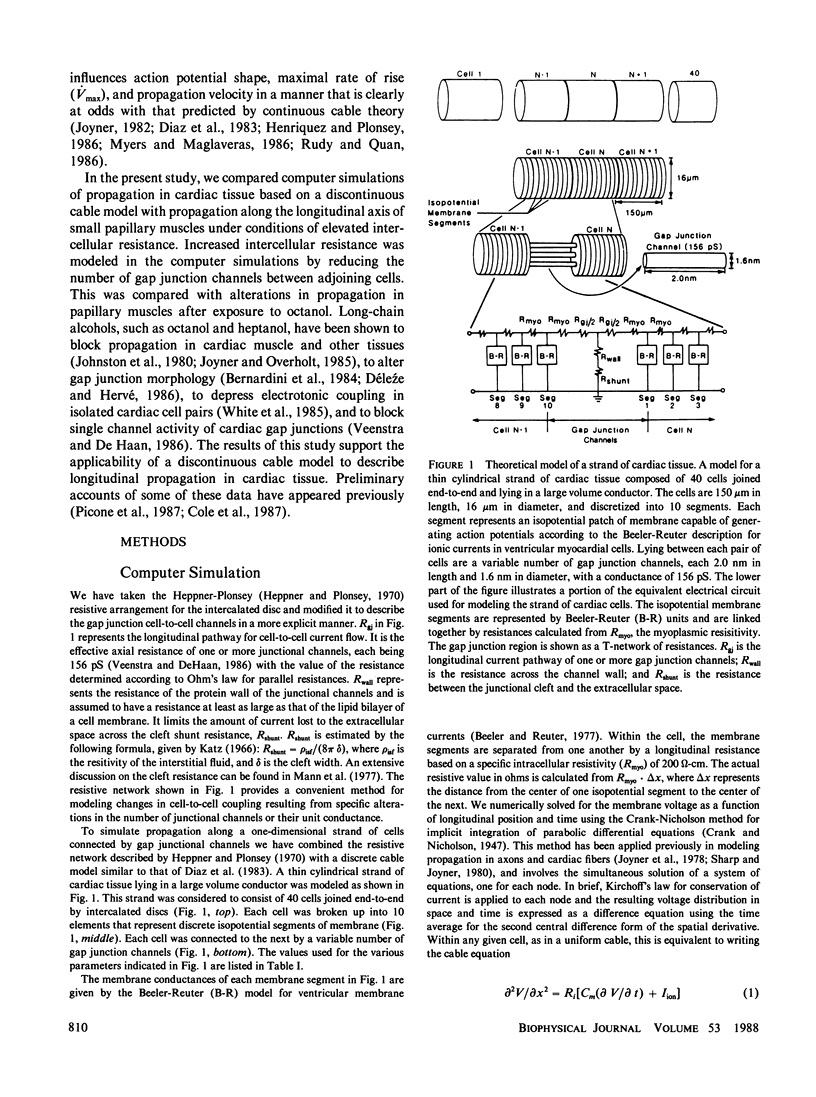
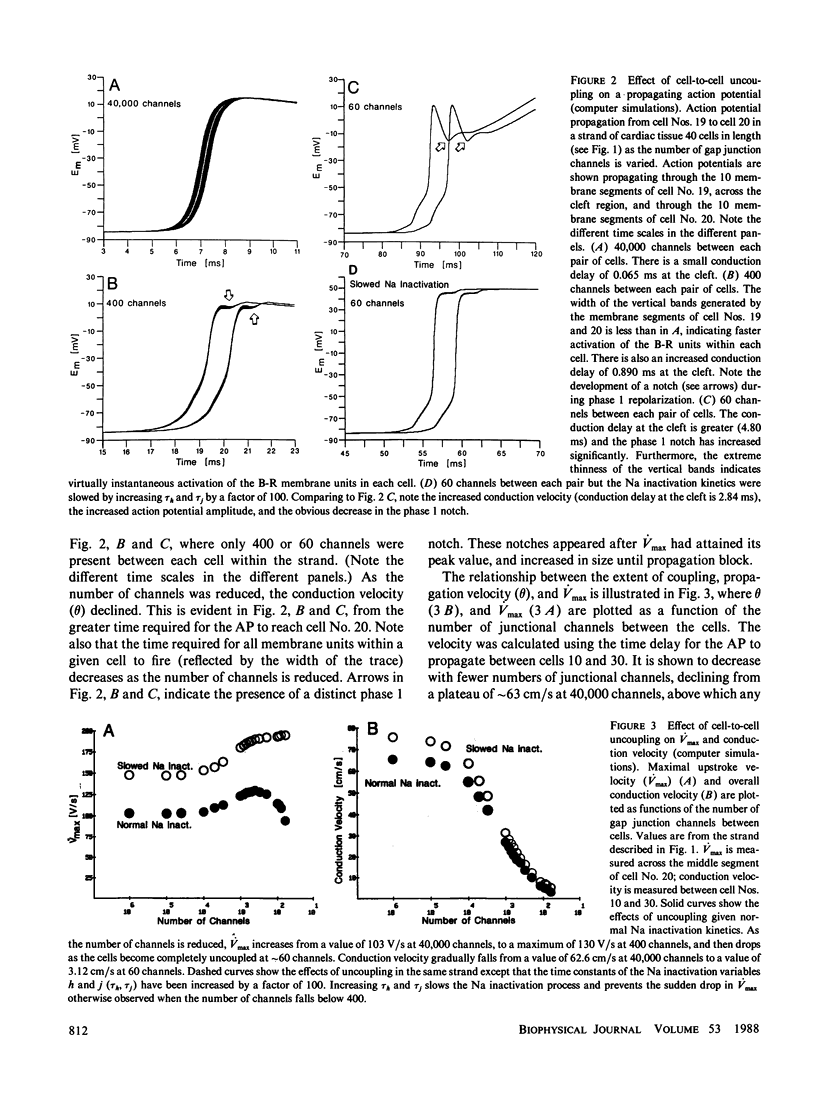
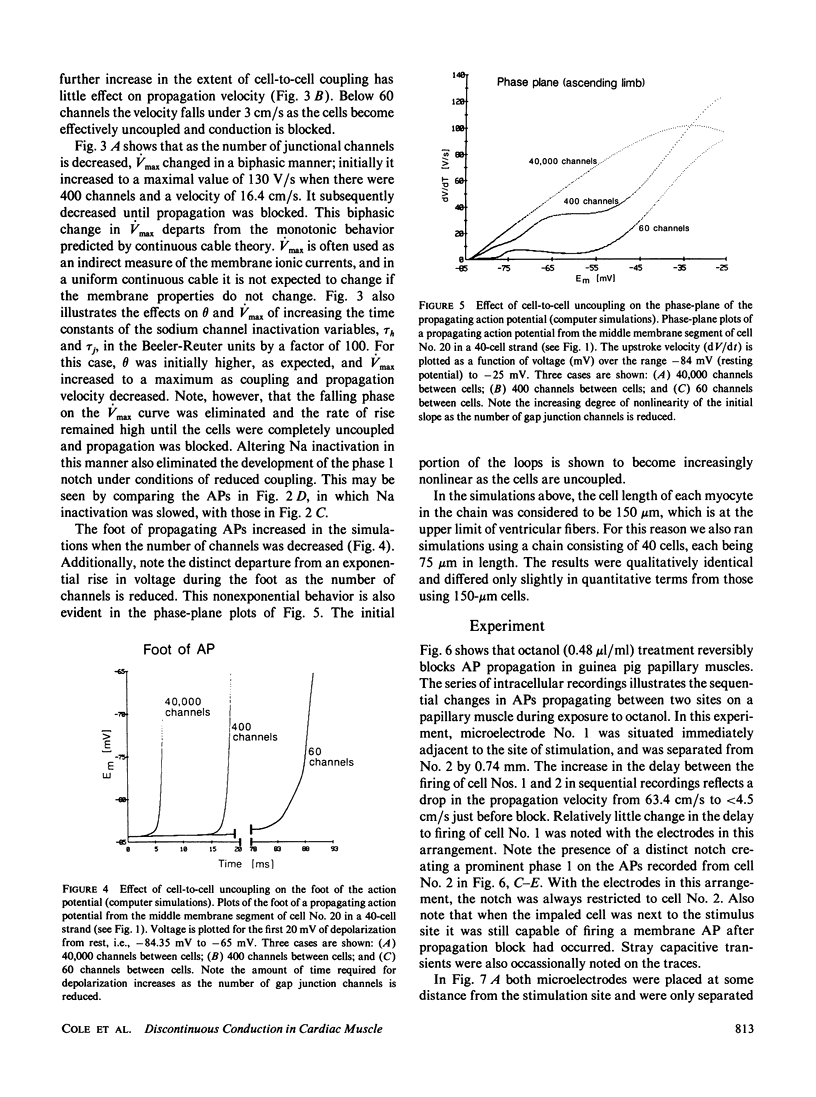
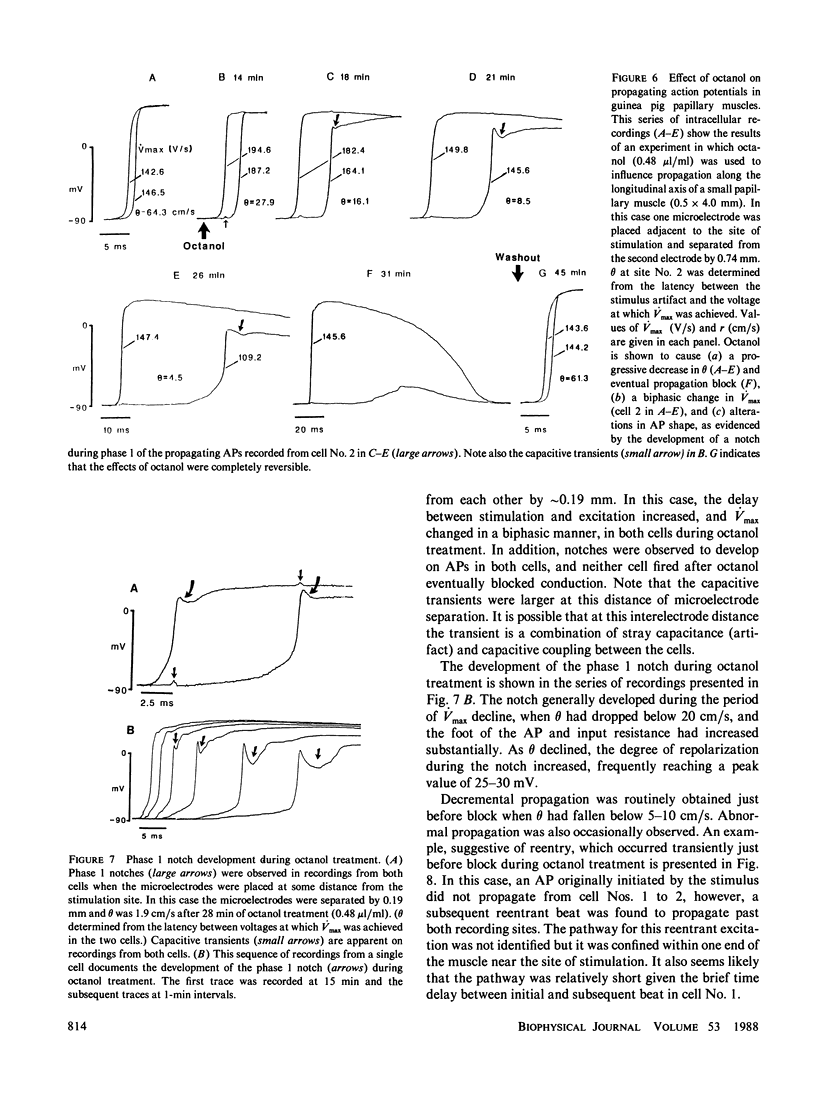
Abstract
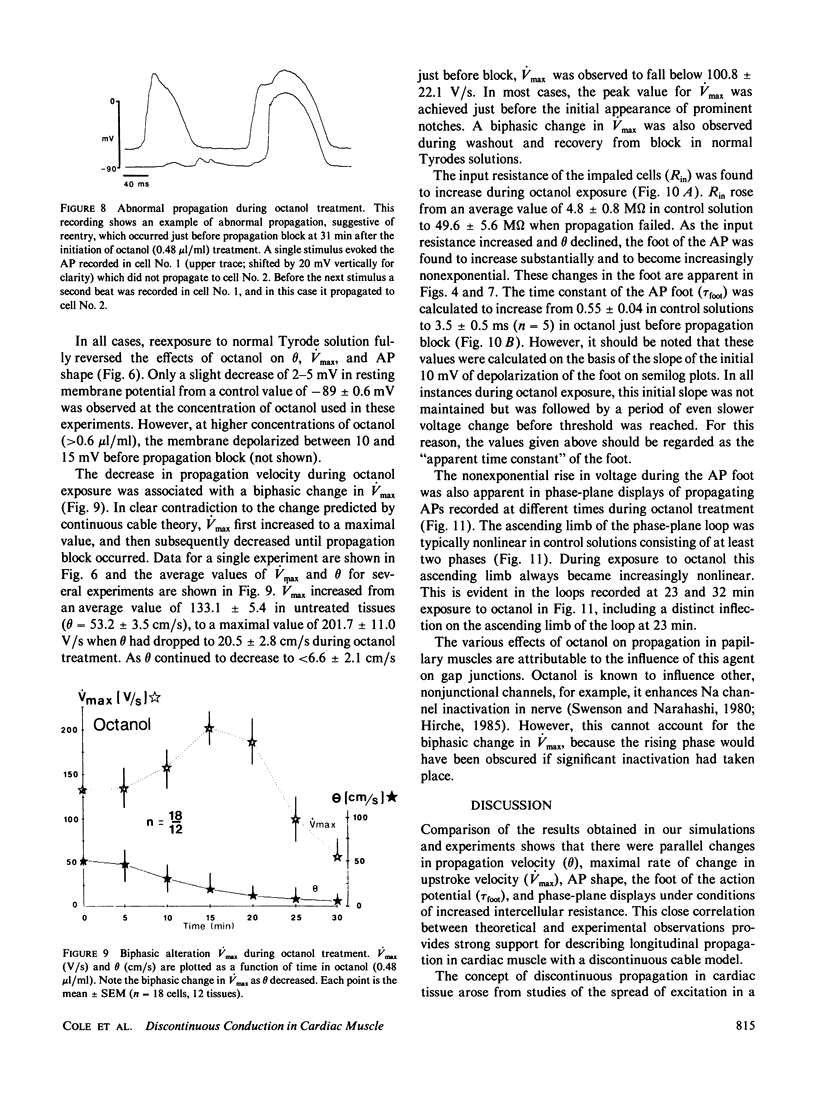
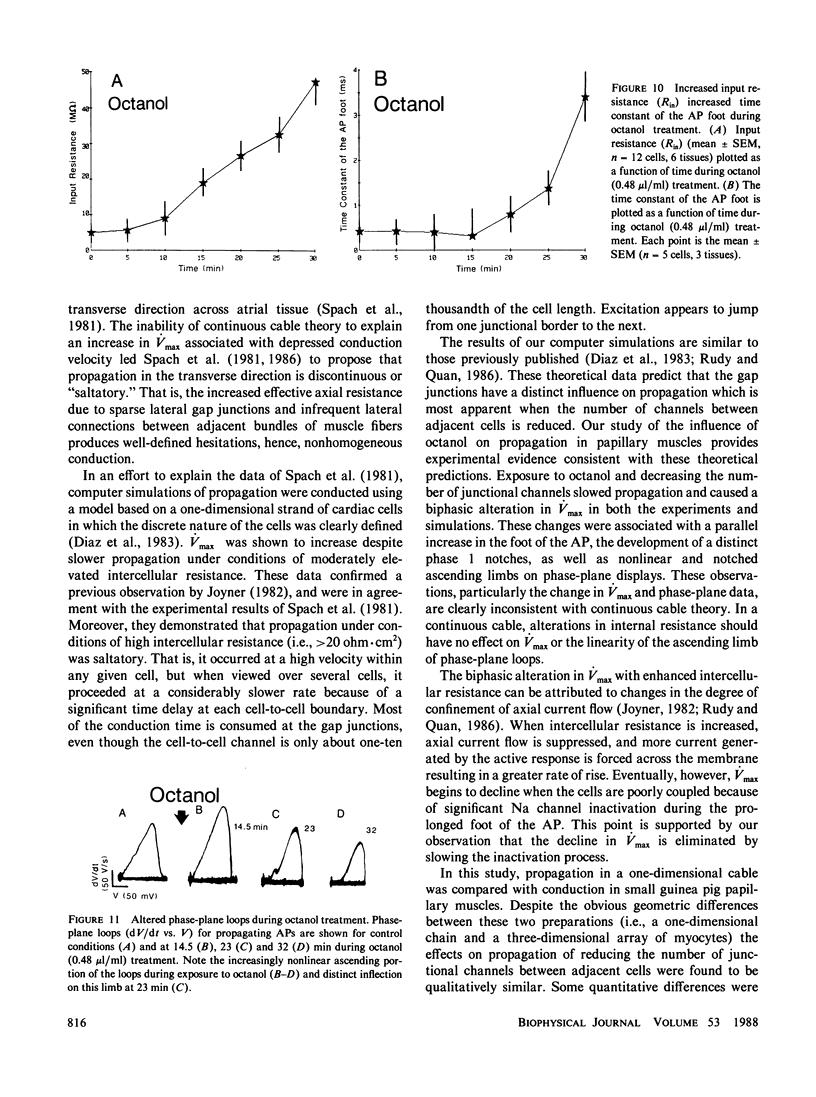
The effects of octanol on longitudinal propagation in guinea pig papillary muscles were measured by intracellular microelectrodes. These data were compared with alterations in conduction induced by stepwise removal of gap junction channels in computer simulations of propagation based on a discontinuous cable model. Octanol reduced the velocity (theta) of propagating action potentials (APs) from 53.2 +/- 3.5 to less than 6.6 +/- 2.1 cm/s before block occurred. The maximal rate of rise (Vmax) changed in a biphasic manner, increasing from 133.1 +/- 5.4 in controls to 201.7 +/- 11.0 V/s when theta was 20.5 +/- 2.8 cm/s, and then declining to less than 58.6 +/- 15.2 V/s just before block. The input resistance and time constant of the AP foot increased, and the ascending limb of phase-plane loops became increasingly nonlinear and notched during octanol treatment. All effects of octanol reversed upon washout. A strand of cardiac tissue was modeled as a discontinuous cable composed of 40 cells, each with 10 isopotential membrane segments described by Beeler-Reuter kinetics, and coupled by a variable number of gap junction channels (156 pS). Decreasing the number of channels from 40,000 to 400 to 60 slowed conduction from 62.6 to 16.4 to 3.1 cm/s. As noted in the experimental data, Vmax increased from 103 to 130 and then fell to less than 96 V/s. The AP foot increased and became nonexponential. Distinct notches developed during phase 1 of the APs at slower propagation velocities in the experiments and simulations. The close similarities between the experimental and theoretical data obtained in this study supports the applicability of a discontinuous cable model for describing longitudinal propagation in the heart.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini G., Peracchia C., Peracchia L. L. Reversible effects of heptanol on gap junction structure and cell-to-cell electrical coupling. Eur J Cell Biol. 1984 Jul;34(2):307–312. [PubMed] [Google Scholar]
- Bonke F. I. Passive electrical properties of atrial fibers of the rabbit heart. Pflugers Arch. 1973 Mar 5;339(1):1–15. doi: 10.1007/BF00586977. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Willems J. The frequency dependent character of the membrane capacity in cardiac Purkyne fibres. J Physiol. 1971 Feb;213(1):85–93. doi: 10.1113/jphysiol.1971.sp009369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc L. Directional differences of impulse spread in trabecular muscle from mammalian heart. J Physiol. 1976 Feb;255(2):335–346. doi: 10.1113/jphysiol.1976.sp011283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello W. C. Passive electrical properties of the atrio-ventricular node. Pflugers Arch. 1977 Oct 19;371(1-2):135–139. doi: 10.1007/BF00580781. [DOI] [PubMed] [Google Scholar]
- Diaz P. J., Rudy Y., Plonsey R. Intercalated discs as a cause for discontinuous propagation in cardiac muscle: a theoretical simulation. Ann Biomed Eng. 1983;11(3-4):177–189. doi: 10.1007/BF02363285. [DOI] [PubMed] [Google Scholar]
- Forbes M. S., Sperelakis N. Intercalated discs of mammalian heart: a review of structure and function. Tissue Cell. 1985;17(5):605–648. doi: 10.1016/0040-8166(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Freygang W. H., Trautwein W. The structural implications of the linear electrical properties of cardiac Purkinje strands. J Gen Physiol. 1970 Apr;55(4):524–547. doi: 10.1085/jgp.55.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSHIKO T., SPERELAKIS N. Prepotentials and unidirectional propagation in myocardium. Am J Physiol. 1961 Nov;201:873–880. doi: 10.1152/ajplegacy.1961.201.5.873. [DOI] [PubMed] [Google Scholar]
- Heppner D. B., Plonsey R. Simulation of electrical interaction of cardiac cells. Biophys J. 1970 Nov;10(11):1057–1075. doi: 10.1016/S0006-3495(70)86352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirche G. Blocking and modifying actions of octanol on Na channels in frog myelinated nerve. Pflugers Arch. 1985 Oct;405(3):180–187. doi: 10.1007/BF00582558. [DOI] [PubMed] [Google Scholar]
- Johnston M. F., Simon S. A., Ramón F. Interaction of anaesthetics with electrical synapses. Nature. 1980 Jul 31;286(5772):498–500. doi: 10.1038/286498a0. [DOI] [PubMed] [Google Scholar]
- Joyner R. W. Effects of the discrete pattern of electrical coupling on propagation through an electrical syncytium. Circ Res. 1982 Feb;50(2):192–200. doi: 10.1161/01.res.50.2.192. [DOI] [PubMed] [Google Scholar]
- Joyner R. W., Overholt E. D. Effects of octanol on canine subendocardial Purkinje-to-ventricular transmission. Am J Physiol. 1985 Dec;249(6 Pt 2):H1228–H1231. doi: 10.1152/ajpheart.1985.249.6.H1228. [DOI] [PubMed] [Google Scholar]
- Joyner R. W., Westerfield M., Moore J. W., Stockbridge N. A numerical method to model excitable cells. Biophys J. 1978 May;22(2):155–170. doi: 10.1016/S0006-3495(78)85481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J. E., Jr, Foley E., Sperelakis N. Resistance and potential profiles in the cleft between two myocardial cells: electrical analog and computer simulations. J Theor Biol. 1977 Sep 7;68(1):1–15. doi: 10.1016/0022-5193(77)90223-5. [DOI] [PubMed] [Google Scholar]
- Sharp G. H., Joyner R. W. Simulated propagation of cardiac action potentials. Biophys J. 1980 Sep;31(3):403–423. doi: 10.1016/S0006-3495(80)85068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach M. S., Dolber P. C. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res. 1986 Mar;58(3):356–371. doi: 10.1161/01.res.58.3.356. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Miller W. T., 3rd, Geselowitz D. B., Barr R. C., Kootsey J. M., Johnson E. A. The discontinuous nature of propagation in normal canine cardiac muscle. Evidence for recurrent discontinuities of intracellular resistance that affect the membrane currents. Circ Res. 1981 Jan;48(1):39–54. doi: 10.1161/01.res.48.1.39. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Shumaker H. K. Phase-plane analysis of cardiac action potentials. J Electrocardiol. 1968;1(1):31–41. doi: 10.1016/s0022-0736(68)80006-8. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Narahashi T. Block of sodium conductance by n-octanol in crayfish giant axons. Biochim Biophys Acta. 1980 Dec 12;603(2):228–236. doi: 10.1016/0005-2736(80)90369-7. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D., DeHaan R. L. Measurement of single channel currents from cardiac gap junctions. Science. 1986 Aug 29;233(4767):972–974. doi: 10.1126/science.2426781. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970 Nov;210(4):1041–1054. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., Spray D. C., Campos de Carvalho A. C., Wittenberg B. A., Bennett M. V. Some electrical and pharmacological properties of gap junctions between adult ventricular myocytes. Am J Physiol. 1985 Nov;249(5 Pt 1):C447–C455. doi: 10.1152/ajpcell.1985.249.5.C447. [DOI] [PubMed] [Google Scholar]
- de Carvalho A. P., Hoffman B. F., de Carvalho M. P. Two components of the cardiac action potential. I. Voltage-time course and the effect of acetylcholine on atrial and nodal cells of the rabbit heart. J Gen Physiol. 1969 Nov;54(5):607–635. doi: 10.1085/jgp.54.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]


