Abstract
Both Neisseria meningitidis and Haemophilus influenzae are capable of mimicking host structures by decorating their lipopolysaccharides with sialic acid. We show that a neuraminidase expressed by Streptococcus pneumoniae (NanA) is able to desialylate the cell surfaces of both these species, which reside in and possibly compete for the same host niche.
Several bacterial species mimic host structures by sialylation of cell surface components (6). Among the major pathogens originating in the human respiratory tract, both Neisseria meningitidis and some isolates of Haemophilus influenzae express a sialyltransferase that adds sialic acid α-2,3 linked to galactose as a terminal structure on their lipopolysaccharide (LPS) (5, 7). The addition of sialic acid promotes survival by decreasing the bactericidal effect of complement through interaction with factor H (15). For N. meningitidis, sialic acid is obtained from cytidine monophospho-N-acetylneuraminic acid (CMP-NANA), which only some strains are able to synthesize (9). For H. influenzae, sialic acid is obtained from environmental sources of 5-acetylneuraminic acid (Neu5Ac) (8).
Streptococcus pneumoniae (the pneumococcus), which is also a common member of the flora of the human upper respiratory tract, has been shown to cleave sialic acid-containing substrates with α-2,3 and α-2,6 linkages to galactose as well as those with α-2,6 linkages to N-acetylgalactosamine (16). The pneumococcus expresses several distinct neuraminidases, including NanA and NanB (1, 2). In some strains, there is also a nanB homolog, nanC, the expression and activity of which have not yet been described. It has been suggested that neuraminidase activity promotes colonization by exposing host cell receptors otherwise covered by sialic acid (19). In this study, we test the hypothesis that an additional target of pneumococcal neuraminidase is sialic acid attached to the cell surface of other members of the nasopharyngeal flora.
N. meningitidis strain N3 or nontypeable H. influenzae strain H122 was grown in the presence or absence of CMP-NANA or Neu5Ac, respectively (Table 1.) Western analysis revealed that growth in the presence of a source of sialic acid corresponded with the loss of the monoclonal antibody (MAb) 3F11 epitope, recognizing lacto-N-neotetraose, a terminal LPS structure to which sialic acid is added in both species (Fig. 1 and 2) (9, 10). The loss of this epitope was associated with the presence of a higher-molecular-weight band in proteinase K-treated lysates in silver-stained Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. Treatment of N. meningitidis (up to 2 × 108 CFU) or H. influenzae (2 × 106 CFU) with purified neuraminidase obtained from Clostridium perfringens (50 mU/ml) (Sigma-Aldrich Co., St. Louis, Mo.) resulted in expression of the MAb 3F11 epitope and loss of the higher-molecular-weight band, confirming that the differences in the LPS were caused by sialylation.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant characteristics |
S. pneumoniae nan genea
|
Source or reference | ||
|---|---|---|---|---|---|
| nanA | nanB | nanC | |||
| N. meningitidis N3 | MC58C3, type B unencapsulated mutant | 12 | |||
| H. influenzae H122 | Nontypeable clinical isolate | This study | |||
| S. pneumoniae | |||||
| P2 | R6, unencapsulatedb mutant of D39 | + | + | − | 20 |
| P394 | Type 4 genome sequence strain | +c | + | + | 14 |
| P1247 | D39ΔnanA | − | + | − | 18 |
| P1252 | Type 21 clinical isolate | + | − | − | This study |
| P1253 | P1252ΔnanA | − | − | − | This study |
The presence of nanB was determined by PCR and Southern hybridization. The presence of nanC was determined by PCR (data not shown). +, present; −, absent.
The presence of capsule had no apparent effect on neuraminidase activity in this study.
Secreted fragment due to frameshift mutation upstream of the C-terminal cell surface-anchoring domain.
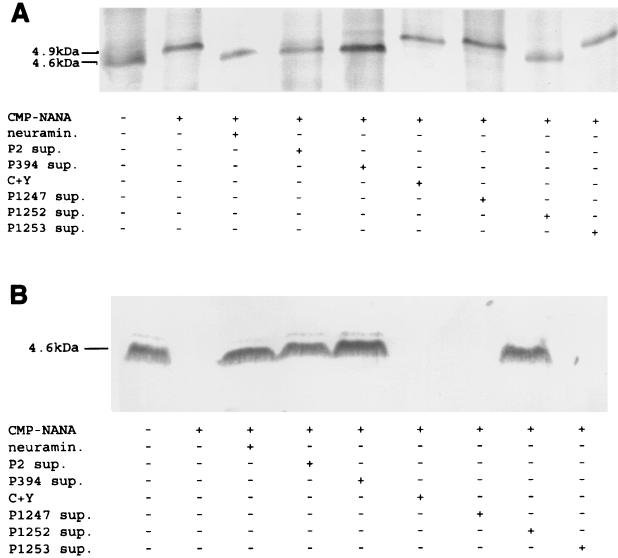
FIG. 1.
Effect of pneumococcal neuraminidase on sialylation of meningococcal LPS. Meningococcal strain N3 was grown up in chemically defined media with or without CMP-NANA (50 μg/ml). A total of 2.0 × 108 CFU were then treated for 30 min at 37°C in C+Y medium with or without C. perfringens neuraminidase (neuramin.; 50 mU/ml) or supernatant (sup.) from 108 CFU of the S. pneumoniae strain indicated (13, 17). The bacterial pellet was incubated with proteinase K at 65°C and then separated in Tricine-SDS-PAGE gels. LPS was visualized with a modified silver stain (A) or transferred to a nitrocellulose membrane and immunoblotted with MAb 3F11 (B).
FIG. 2.
Effect of pneumococcal neuraminidase on sialylation of H. influenzae LPS. H. influenzae strain H122 was grown on brain heart infusion agar supplemented with 1.5% Fildes enrichment (Difco Labs, Detroit, Mich.) with or without Neu5Ac (100 μg/ml). A total of 2.0 × 106 CFU were then treated for 30 min at 37°C in C+Y medium with or without C. perfringens neuraminidase (neuramin.; 50 mU/ml) or supernatant (sup.) from 1.0 × 108 CFU of the S. pneumoniae strain indicated. The bacterial pellet was lysed by treatment at 100°C for 5 min and then separated in Tricine-SDS-PAGE gels. LPS was visualized with a modified silver stain (data not shown) or transferred to a nitrocellulose membrane and immunoblotted with MAb 3F11.
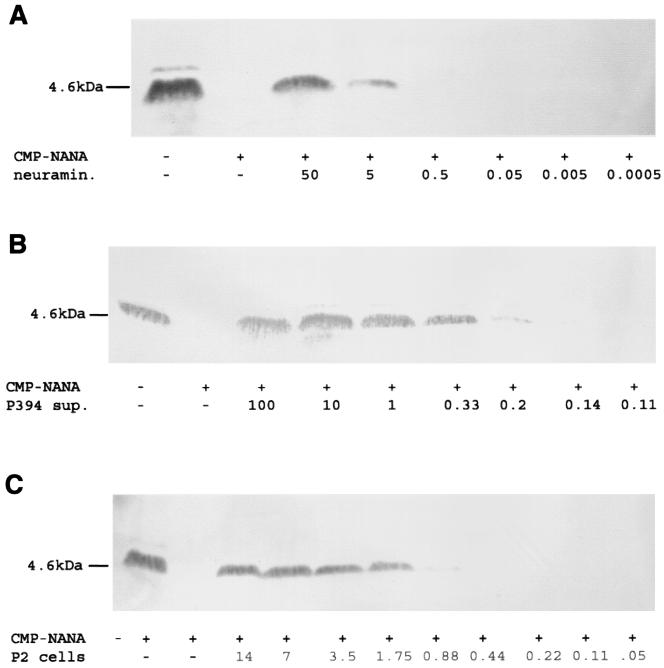
The effect of the pneumococcus in vitro was tested by incubation of N. meningitidis or H. influenzae under conditions allowing for LPS sialylation with culture supernatants of S. pneumoniae grown to the mid-log phase in C+Y medium (17). Incubation of N3 or H122 for 30 min at 37°C with the supernatant fraction of growth medium from pneumococcal strain P2 or P394 (108 CFU/ml) resulted in loss of sialylation (Fig. 1 and 2). No effect was seen in control supernatants containing the C+Y growth medium alone. The addition of CMP-NANA (50 μg/ml) during incubation of N3 with the pneumococcal supernatants had no effect on desialylation, suggesting that under these conditions, the activity of the neuraminidase was more efficient than that of the meningococcal sialyltransferase (data not shown). The ability to desialylate the LPS of N3 and H122 was noted in culture supernatant from strain P1252, but not that from P1247 or P1253, indicating that nanA is required for this activity (Fig. 1 and 2). The lack of activity in P1247 cells or culture supernatant, even when tested after growth to the stationary phase, when NanB expression is optimal, suggests that nanB does not contribute to the desialylation of the LPS (data not shown) (3). The neuraminidase activities of strains P2 (cell fraction) and P394 (culture supernatant fraction) were quantified by comparison to that of purified C. perfringens neuraminidase in serial dilutions (Fig. 3.) The results demonstrate that 5 mU of neuraminidase activity was sufficient for complete desialylation of 2 × 108 meningococci. The neuraminidase activity for strain P394 was estimated at 12 mU per supernatant fraction for 106 cells and 0.3 mU/106 cells for the cell fraction of strain P2. Thus, we estimate that the supernatant derived from one P394 cell is sufficient to desialylate about 1,000 meningococci under these conditions. In contrast, the activity of one P2 cell was sufficient to desialylate only about 25 meningococci. P394 contains a frameshift upstream of the C-terminal cell wall LPXTGX-anchoring motif in nanA, explaining why >95% of the enzyme activity was found in the culture supernatant rather than the cell fraction. Since the assay conditions would predict more efficient access of the enzyme to its target in the secreted form, the secretion of NanA in P394 could account for its higher level of activity. For P2, the neuraminidase activity was divided between the culture supernatant (28% of activity) and cell fractions (72% of activity), indicating partial release of the enzyme (data not shown).
FIG. 3.
Western analysis quantifying the neuraminidase activity of S. pneumoniae necessary for removal of sialic acid from meningococcal LPS. The absence of the MAb 3F11-reactive band indicates LPS sialylation. Meningococcal strain N3 grown with or without CMP-NANA (50 μg/ml) was treated as described above with purified C. perfringens neuraminidase (neuramin.) at the final concentration indicated (milliunits per milliliter) (A) or culture supernatant (sup.) from P394 cells at the cell density indicated (106 CFU) (B) or the cell fraction of P2 cells at the cell density indicated (107 CFU) (C).
We have previously shown that high concentrations of hydrogen peroxide produced by the aerobic metabolism of the pneumococcus may be inhibitory or bactericidal in vitro to other species that reside in the same environment, including H. influenzae and N. meningitidis (14). The findings in this study describe a second mechanism whereby S. pneumoniae could interfere with the biology of potential competitors. In the case of desialylation of the cell surfaces of other members of the microflora, the pneumococcus appears to be specifically targeting a mechanism involving bacterial adaptation to its host. It remains to be determined whether interspecies competition occurs in the heavily colonized human upper respiratory tract in which each of the three species examined here resides. In this regard, several previous reports suggest that the pneumococcus may have inhibitory effects on H. influenzae in the natural host. During exacerbations of chronic bronchitis, H. influenzae was isolated less frequently during periods when the pneumococcus was present compared to periods when it was absent (11). Recent results from a randomized double-blind trial of the pneumococcal conjugate vaccine showed that a decrease in the incidence of carriage and otitis media caused by pneumococcal types in the vaccine was associated with an 11% increase in disease due to H. influenzae in the vaccine group (4). Disease caused by the meningococcus is less common, and we are not aware of a similar inverse association with the pneumococcus having been reported. Clinical observations about S. pneumoniae and H. influenzae, however, point out the need to understand the potential interactions of microorganisms, since manipulation of the human microflora may lead to unanticipated problems.
Acknowledgments
We thank R. Rest for guidance with using the meningococcus, M. Apicella for supplying MAb 3F11, and T. DeMaria, C. Dowson, and A. Whatmore for providing strains.
This work was supported by grants from the Public Heath Service (AI38436 and AI44231).
Editor: V. J. DiRita
REFERENCES
- 1.Berry, A. M., R. A. Lock, and J. C. Paton. 1996. Cloning and characterization of nanB, a second Streptococcus pneumoniae neuraminidase gene, and purification of the NanB enzyme from recombinant Escherichia coli. J. Bacteriol. 178:4854-4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cámara, M., G. J. Boulnois, P. W. Andrew, and T. J. Mitchell. 1994. A neuraminidase from Streptococcus pneumoniae has the features of a surface protein. Infect. Immun. 62:3688-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Saizieu, A., U. Certa, J. Warrington, C. Gray, W. Keck, and J. Mous. 1998. Bacterial transcript imaging by hybridization of total RNA to oligonucleotide arrays. Nat. Biotechnol. 16:45-48. [DOI] [PubMed] [Google Scholar]
- 4.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen et al. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert, M., D. Watson, A. Cunningham, M. Jennings, N. Young, and W. Wakarchuk. 1996. Cloning of the lipooligosaccharide alpha-2,3-sialyltransferase from the bacterial pathogens Neisseria meningitidis and Neisseria gonorrhoeae. J. Biol. Chem. 271:28271-28276. [DOI] [PubMed] [Google Scholar]
- 6.Harvey, H., W. Swords, and M. Apicella. 2001. The mimicry of human glycolipids and glycosphingolipids by the lipooligosaccharides of pathogenic Neisseria and Haemophilus. J. Autoimmun. 16:257-262. [DOI] [PubMed] [Google Scholar]
- 7.Hood, D., A. Cox, M. Gilbert, K. Makepeace, S. Walsh, M. Deadman, A. Cody, A. Martin, M. Mansson, E. Schweda, J. Brisson, J. Richards, E. Moxon, and W. Wakarchuk. 2001. Identification of a lipopolysaccharide alpha-2,3-sialyltransferase from Haemophilus influenzae. Mol. Microbiol. 39:341-350. [DOI] [PubMed] [Google Scholar]
- 8.Hood, D., K. Makepeace, M. Deadman, R. Rest, P. Thibault, A. Martin, J. Richards, and E. Moxon. 1999. Sialic acid in the lipopolysaccharide of Haemophilus influenzae: strain distribution, influence on serum resistance and structural characterization. Mol. Microbiol. 33:679-692. [DOI] [PubMed] [Google Scholar]
- 9.Mandrell, R. E., J. J. Kim, C. M. John, B. W. Gibson, J. V. Sugai, M. A. Apicella, J. M. Griffiss, and R. Yamasaki. 1991. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J. Bacteriol. 173:2823-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandrell, R. E., R. McLaughlin, Y. A. Kwaik, A. Lesse, R. Yamasaki, B. Gibson, S. M. Spinola, and M. A. Apicella. 1992. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialylated. Infect. Immun. 60:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.May, R. J. 1954. Pathogenic bacteria in chronic bronchitis. Lancet ii:839-842. [DOI] [PubMed]
- 12.McGuinness, B., I. Clarke, P. Lambden, A. Barlow, J. Poolman, D. Jones, and J. Heckels. 1991. Point mutation in meningococcal porA gene associated with increased endemic disease. Lancet 337:514-517. [DOI] [PubMed] [Google Scholar]
- 13.Morse, S., and L. Bartenstein. 1980. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can. J. Microbiol. 26:13-20. [DOI] [PubMed] [Google Scholar]
- 14.Pericone, C. D., K. Overweg, P. W. M. Herman, and J. N. Weiser. 2000. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect. Immun. 68:3990-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ram, S., A. K. Sharma, S. D. Simpson, S. Gulati, D. P. McQuillen, M. K. Pangburn, and P. A. Rice. 1998. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J. Exp. Med. 187:743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scanlon, K., W. Diren, and R. Glew. 1989. Purification and properties of Streptococcus pneumoniae neuraminidase. Enzyme 41:143-150. [DOI] [PubMed] [Google Scholar]
- 17.Tomasz, A. 1964. A chemically defined medium for Streptococcus pneumoniae. Bacteriol. Proc. 64:29. [Google Scholar]
- 18.Tong, H. H., L. E. Blue, M. A. James, and T. F. DeMaria. 2000. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase-deficient mutant in nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect. Immun. 68:921-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong, H. H., M. James, I. Grants, X. Liu, G. Shi, and T. F. DeMaria. 2001. Comparison of structural changes of cell surface carbohydrates in the eustachian tube epithelium of chinchillas infected with a Streptococcus pneumoniae neuraminidase-deficient mutant or its isogenic parent strain. Microb. Pathog. 31:309-317. [DOI] [PubMed] [Google Scholar]
- 20.Weiser, J. N., Z. Markiewicz, E. I. Tuomanen, and J. H. Wani. 1996. Relationship between phase variation in colony morphology, intrastrain variation in cell wall physiology, and nasopharyngeal colonization by Streptococcus pneumoniae. Infect. Immun. 64:2240-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]