Abstract
The human pathogen Streptococcus pyogenes primarily infects the upper respiratory tract and skin, but occasionally it disseminates and causes severe invasive disease with high mortality. This study revealed that the activity of extracellular EndoS, which hydrolyzes the functionally important N-linked oligosaccharides on opsonizing immunoglobulin G (IgG), contributes to increased survival of S. pyogenes in human blood ex vivo. The inability to kill the bacteria is due to reduced binding of IgG to Fc receptors and impaired classical pathway-mediated activation of complement. In addition, the activity of extracellular SpeB, which cleaves IgG into Fc and Fab fragments, also increases bacterial survival. This suggests that S. pyogenes expresses two enzymes, EndoS and SpeB, which modulate IgG by different mechanisms in order to evade the adaptive immune system.
A prerequisite for a pathogen to successfully invade and colonize a host is to escape recognition by the immune system. Many host defense mechanisms are involved in the battle against bacterial infections, and two significant events are opsonization and phagocytosis. These events involve antigen recognition, complement deposition, and phagocytic cells that engulf and kill the microorganism after recognition by host receptors for the Fc portion of immunoglobulins, Fc receptors (FcR), and complement receptors (CR1 and CR3). In this process, the human host relies on the adaptive immune response that produces opsonizing antibodies directed towards surface-exposed antigens on the microorganism.
Human immunoglobulin G (IgG) is composed of two identical light chains and two identical heavy chains. These chains form three independent protein domains connected through the flexible and protease-sensitive hinge region. The two Fab fragments of IgG bind antigen, while the Fc region is the site of interaction with a number of effector molecules, including the complement protein C1q. The Fc region also interacts with phagocytic cells, such as monocytes, macrophages, and neutrophils. A conserved complex biantennary oligosaccharide is attached to each Asn297 in the CH2 domain (29, 36). These oligosaccharides are located in the cavity between the CH2 domains and are thought to stabilize the molecule (10). The structural and biological significance of these oligosaccharides has been extensively investigated (11, 28, 30, 38, 43).
Many molecules involved in the innate and adaptive immune system are glycoproteins (31). Consequently, the immune evasion strategies used by pathogens may include interfering with oligosaccharides on glycoproteins. Potential targets could be the N-linked oligosaccharides on the heavy chains of immunoglobulins, which are known to play a role in effector functions, including complement activation and FcR binding on effector cells (4, 28).
Human pathogens have evolved many strategies to evade immune recognition. Examples include the IgA proteases found in many bacterial species (18, 23, 27). These IgA proteases are highly specific extracellular proteins that cleave IgA in the hinge region into Fc and monomeric Fab fragments, resulting in impairment of IgA effector functions (19). Specific IgG proteases are not common, but IgG-degrading proteases are produced by a variety of human pathogens, such as Pseudomonas aeruginosa (14), Prevotella intermedia, and Prevotella nigrescens (15), as well as by the helminth parasite Paragonimus westermani (32).
Streptococcus pyogenes, which is a significant human pathogen, causes infections such as impetigo, scarlatina, and pharyngitis. It also causes severe invasive diseases like necrotizing fasciitis and sepsis (9). S. pyogenes secretes a number of proteins that potentially can affect the human host. One abundant protein is the well-characterized streptococcal cysteine proteinase SpeB (17). Recently, the IgG-hydrolyzing activity of SpeB, which cleaves human IgG into Fc and monomeric Fab fragments, was described (6). In the same report, a novel extracellular enzyme from S. pyogenes, designated EndoS, was identified and characterized. EndoS hydrolyzes the conserved N-linked oligosaccharides on the heavy chains of human IgG. Interestingly, EndoS hydrolyzes only the glycan on native IgG and is the first enzyme from a bacterial pathogen with this unique specificity (5, 6).
Enzymatic activity against oligosaccharide structures on IgG or cleavage in the hinge region could have implications for IgG functionality in response to a streptococcal infection. In this study we showed that two extracellular enzymes from S. pyogenes, EndoS and SpeB, alter opsonizing antibodies, resulting in increased bacterial survival in human blood. Thus, expression of these enzymes might help the bacteria evade an attack by the immune system.
MATERIALS AND METHODS
Recombinant expression of EndoS.
EndoS was expressed in Escherichia coli by using the glutathione S-transferase (GST) gene fusion system (Amersham-Pharmacia Biotech, Uppsala, Sweden) as described previously (5). A 2,929-bp PCR product including bases 304 to 3232 of the ndoS sequence was amplified from S. pyogenes genomic DNA by using primer 5′-ACT-GGG-ATC-CCG-GAG-GAG-AAG-ACT-3′ with a BamHI site (underlined) and primer 5′-TTA-ATC-TCG-AGG-TTG-CTA-TCT-AAG-3′ with an XhoI site (underlined). This fragment was digested with BamHI and XhoI and ligated into pGEX-5X-3, generating plasmid pGEXndoS, which was used to transform E. coli BL21(DE3)pLysS. BL21(DE3)pLysS/pGEXndoS was induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside. Cleavage of GST from EndoS with factor Xa and removal of factor Xa were performed as described previously (5).
SpeB and recombinant EndoS (rEndoS) treatment of human IgGops.
To obtain immune sera, blood samples from healthy individuals were screened for the ability to kill the S. pyogenes AP1 strain. For purification of opsonizing IgG (IgGops), serum was isolated from the volunteers in whose blood S. pyogenes did not multiply. The IgG was purified by affinity chromatography by using HiTrap protein G-Sepharose according to the manufacturer's instructions (Amersham-Pharmacia Biotech). Purified IgG was dialyzed against phosphate-buffered saline (PBS) prior to use.
SpeB was purified from strainAP1 cells as described previously (3). The S. pyogenes strain AP1 used in this study is a serotype M1 strain and has been well characterized in terms of expression of cysteine proteinase (7), M1 protein (2), the M-like IgG-binding protein H (1), and IdeS (41). For SpeB treatment, 150 μg of purified IgG was incubated for 24 h at 37°C in 120 μl of PBS with 50 μg of SpeB and 0.2 mM dithiothreitol (DTT) or with DTT alone. For rEndoS treatment, 200 μg of purified IgG was incubated for 24 h with 10 μg of rEndoS or with PBS alone. SpeB-treated IgG was analyzed by nonreducing sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis (PAGE), and rEndoS-treated IgG was analyzed by SDS-PAGE or lectin blot analysis with Galanthus nivalis lectin as described previously (6). Briefly, after separation, the IgG was electroblotted onto a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.). The membrane was blocked with Tris-buffered saline containing 0.1% Tween 20 (TBST) and incubated with 0.5 μg of biotinylated G. nivalis lectin (Vector Laboratories, Burlingame, Calif.) per ml. After the membrane was washed in TBST, it was incubated with 1 μg of peroxidase-labeled streptavidin (Vector Laboratories) per ml. The membrane was washed in TBST and was developed by using 1.25 mM 5-amino-2,3-dihydro-1,4-phthalazinedione, 0.22 mM p-coumaric acid, and 0.09% H2O2 (Sigma, St. Louis, Mo.) according to the Immunoprint method (25). Subsequently, the membrane was exposed on Cronex X-ray film (Sterling Diagnostic Imaging, Newark, Del.).
Opsonophagocytic killing assays.
Opsonophagocytic killing assays were performed by using an S. pyogenes isolate belonging to the M1 serotype. Bacteria were cultured in Todd-Hewitt broth supplemented with 0.2% yeast extract (Difco, Detroit, Mich.) at 37°C in the presence of 5% CO2. The overnight culture was diluted 1:30, and at an optical density at 620 nm of 0.15 the culture was further diluted 1:104. One hundred microliters (approximately 300 to 400 CFU/ml) was inoculated into 1 ml of fresh heparinized human blood from three different donors. Before inoculation, the blood was supplemented with 100 μg of IgG treated with SpeB, DTT alone, rEndoS, PBS, or (as a control) heparinized blood with nothing added. Growth of the bacteria was monitored by plating dilutions of the mixtures on Todd-Hewitt agar plates supplemented with 0.2% yeast extract. At 180 min the concentrations of bacteria in the different experimental preparations varied between 3.1 × 104 and 4.4 × 105 CFU/ml. Thus, the multiplication factor was calculated by dividing the number of colonies after 180 min of incubation by the number of colonies at time zero.
MBL-deficient sera.
All sera were stored in aliquots at −80°C. Fresh serum from a donor that belonged to blood group AB and was Rh positive was purchased from the University Hospital of Lund. This serum was found to be mannan-binding lectin (MBL) deficient, as determined by an enzyme-linked immunosorbent assay (ELISA) (MBL concentration, <15 μg/liter), and the deficiency was confirmed (MBL concentration, 4.4 μg/liter) by a time-resolved immunofluorometric assay (34). C1q, factor D, and properdin were removed from the MBL-deficient serum, and the serum was reconstituted with purified C1q, factor D, and properdin (12, 33) and with MBL-associated serine protease complexes (42).
C3 deposition.
ELISA plates (Nunc Immunoplates; Maxisorp; A/S Nunc, Kamstrup, Denmark) were coated overnight at 4°C with native IgG or rEndoS-treated IgG at a concentration of 1 μg per well in carbonate buffer (50 mmol/liter; pH 9.5). Three wells (two coated wells and one uncoated well) were used to analyze each sample. After the wells were washed with PBS, all of the wells were blocked for 2 h at room temperature with Veronal-buffered saline containing Ca2+ (0.15 mmol/liter), Mg2+ (0.5 mmol/liter), and 0.1% gelatin. Purified complement proteins were added to the MBL-deficient serum from which C1q, factor D, and properdin had been removed by using C1q at a concentration of 17.5 mg/ml (i.e., 25% of the normal concentration), factor D at a concentration of 1.0 mg/ml (i.e., physiological concentration), and properdin at a concentration of 25 mg/liter (i.e., physiological concentration) (8). MBL was added at a concentration of 2 mg/liter, a fairly high concentration (35). Serum samples were diluted in Veronal-buffered saline containing Ca2+ and Mg2+ and were added at a final concentration of 25% to the assay mixtures. Incubation was carried out for 30 min at 37°C. After the preparations were washed with PBS containing 0.05% Tween, C3 deposition was measured with rabbit anti-C3c antibodies (DAKO A/S, Glostrup, Denmark) conjugated with alkaline phosphate (Sigma). The plates were incubated at room temperature for 2 h. The reaction was visualized by adding 50 μl of a 1-mg/ml p-nitrophenylphosphate (Sigma) solution in diethanolamine (ICN Biochemicals, Inc., Aurora, Ohio) (pH 9.8) to each well. After 30 min of incubation in the dark at room temperature, the enzymatic reaction was measured at 405 nm with a Multiskan Plus photometer (Labsystems Ltd., Helsinki, Finland). For each serum sample analyzed, the background absorbance in the gelatin-blocked wells without IgG was subtracted from the mean absorbance of the wells coated with native IgG or rEndoS-treated IgG. For quantitative expression of C3 deposition, a reference curve was prepared based on direct adsorption of purified C3 (37) onto the ELISA plates. The C3 concentration in a preparation was determined by rocket electrophoresis (16) with rabbit anti-C3c in the gel and with a commercial calibrator as a reference (human serum protein calibrator code no. X0908; DAKO).
Flow cytometry.
The promonocytic cell line U937 was maintained at 37°C in the presence of 5% CO2 in RPMI 1640 medium supplemented with Glutamax-I, 10% heat-inactivated fetal calf serum, 50 U of penicillin per ml, and 50 μg of streptomycin (Gibco BRL, Bethesda, Md.) per ml. Cells were washed once in Hanks' balanced salt solution (Gibco BRL), and 106 cells were pelleted in each well of a round-bottom 96-well plate (Becton Dickinson, Franklin Lakes, N.J.). The pellets were resuspended in 100 μl of fluorescence-assisted cell sorting buffer (PBS containing 2% fetal calf serum and 0.05% NaN3) containing 1 μg of untreated IgG per ml or 1 μg of rEndoS-treated IgG per ml, incubated at 20°C for 10 min, and washed once. The cells were then resuspended in 100 μl of fluorescence-assisted cell sorting buffer containing 1 μg of the fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 fragment of rabbit anti-human IgG specific for γ-chains (DAKO) per ml, incubated at 20°C for 10 min, washed once, and immediately analyzed with a FACSCalibur flow cytometer (Becton Dickinson). In the experiments in which EndoS-treated IgG was used together with U937 cells, both GST-EndoS-treated IgG and rEndoS-treated IgG were examined. When GST-EndoS-treated IgG was used, excess GST-EndoS was removed by using glutathione-Sepharose and was analyzed by using G. nivalis lectin as described previously (6) before addition to U937 cells. Comparable results were obtained when GST-EndoS was removed by affinity chromatography (see Fig. 3; also data not shown). Thus, we concluded that EndoS per se did not have any effect on U937 cells.
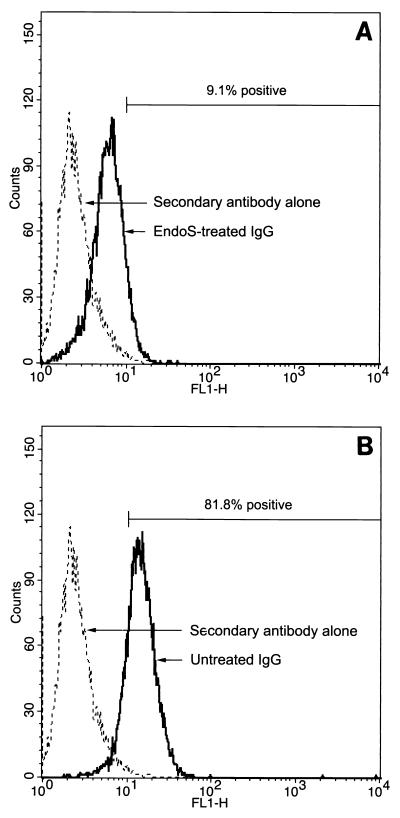
FIG. 3.
rEndoS-treated IgG binds poorly to leukocytes. Binding of rEndoS-treated (A) and intact IgG (B) to U937 cells was analyzed by flow cytometry with FITC-labeled rabbit F(ab′)2 fragments directed against human IgG. Cells with a fluorescence of >10 were considered positive for IgG binding. The percentages of positive cells are mean values from three independent experiments. As a control, cells were incubated with secondary antibody alone.
RESULTS
EndoS hydrolysis of IgG impairs killing of bacteria in blood due to diminished FcR binding and complement activation.
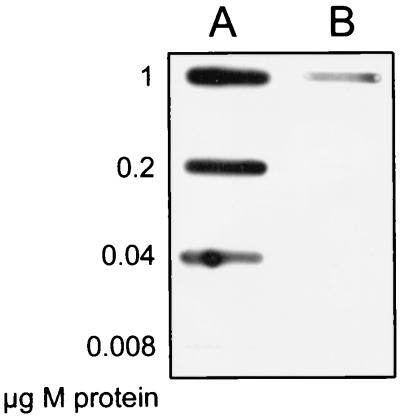
The enzymatic activity of EndoS, which hydrolyzes the β-1,4-di-N-acetylchitobiose core of the N-linked glycan of human IgG, during streptococcal growth in whole blood was investigated. By using the opsonophagocytic killing assay described by Lancefield (20), the capacity of EndoS to alter the functionality of an opsonizing antibody was examined. In this analysis, polyclonal IgG containing opsonizing antibodies was isolated from a healthy donor. The specificity of the IgGops was compared with that of control IgG for reactivity against M1 protein purified from the indicator strain used by immunoblotting. This revealed that the IgGops reacted with M1 protein, while the control IgG from the nonimmune donors bound M1 to a significantly lesser extent (Fig. 1). The IgGops was treated with purified rEndoS before addition to whole blood from three different donors lacking opsonizing antibodies against S. pyogenes. Bacteria were inoculated into the whole blood-IgG mixtures, and bacterial survival was monitored over time. rEndoS activity on IgG was confirmed by SDS-PAGE analysis, which showed that the rEndoS-treated IgG migrated as a protein that was approximately 4 kDa smaller than the untreated IgG protein (Fig. 2A). Removal of the oligosaccharide was confirmed by using G. nivalis lectin, which recognizes the α-1,3 mannose residue on the γ-chain (Fig. 2A).
FIG. 1.
Purified IgGops binds to purified M1 protein. Serial dilutions of M1 protein were applied to a membrane in a slot-binding experiment, followed by incubation with IgGops (lane A) or nonopsonizing IgG (lane B). IgG was subsequently detected with peroxidase-labeled protein A.
FIG. 2.
rEndoS hydrolysis of IgGops promotes bacterial growth in human blood. (A) SDS-PAGE and lectin blot analysis of IgG incubated with (+) or without (−) rEndoS. Lanes A and C, IgG incubated with rEndoS; lanes B and D, IgG incubated with PBS alone. (B) Representative results of opsonophagocytic killing assays performed with rEndoS-treated IgG from a donor. IgG treated with rEndoS (rEndoS-treated IgG) or IgG treated with PBS (intact IgG) was added to human blood. Alternatively, no IgG or rEndoS alone was added. Bacterial growth was monitored after 3 h of incubation. The mean multiplication factor (n = 5) and standard deviations are shown.
Subsequently, the rEndoS-treated IgGops were analyzed in the opsonophagocytic killing assay. This assay revealed that after incubation in human blood for 3 h, untreated IgGops inhibited growth to a significantly greater extent than rEndoS-treated IgGops inhibited growth. Addition of rEndoS alone without any antibodies did not influence growth (Fig. 2B). These data show that pretreatment of human IgGops with rEndoS leads to significantly increased bacterial survival, demonstrating the importance of the oligosaccharides for IgG function.
IgG oligosaccharides are important for stability and function of the molecule. To determine if reduced killing of bacteria is due to impaired IgG binding to FcR on leukocytes, rEndoS-treated IgG was analyzed for binding to promonocytic U937 cells. Flow cytometry with FITC-labeled rabbit F(ab′)2 directed against human IgG was used to detect IgG binding to the cells. Three separate experiments revealed that 81.8% ± 2% of the cells incubated with intact IgG were positive for IgG binding, while only 9.1% ± 0.2% of the cells incubated with rEndoS-treated IgG were positive (Fig. 3). This demonstrates that the N-linked oligosaccharides of IgG are important for the interaction with FcR on leukocytes.
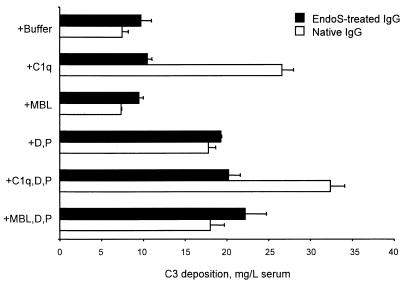
Another possible explanation for the impaired opsonizing activity is that oligosaccharides are important for activation of complement through one of its three pathways, the classical, the alternative, and the MBL pathways (40). MBL binds directly to certain carbohydrate structures and has an opsonizing function per se in addition to its capacity to trigger complement activation through the MBL pathway (26). The activation of complement was assessed by comparing the capacities of native IgG and rEndoS-treated IgG to support deposition of serum C3 in an ELISA system. Microtiter plate wells were coated with intact native IgG or rEndoS-treated IgG and were then incubated with serum at 37°C for 30 min. C3 deposition was measured with enzyme-conjugated rabbit anti-C3c. MBL-deficient serum from which C1q, factor D, and properdin had been removed was used as the complement source. Purified complement proteins were added to the serum to differentiate among the complement pathways (Fig. 4). Quite clearly, rEndoS treatment destroyed the capacity of IgG to activate the classical pathway. There was no evidence of MBL pathway activation, and both IgG preparations supported alternative pathway-mediated C3 deposition under the conditions used.
FIG. 4.
Complement activation by native IgG and rEndoS-treated IgG. IgG was coated onto wells of microtiter plates. MBL-deficient serum from which C1q, factor D (D), and properdin (P) were removed was used as a complement source at a 1:4 dilution. Purified complement proteins were added to the serum as indicated on the left. C3 deposition was detected with enzyme-conjugated rabbit anti-C3c and was expressed relative to the amount of undiluted serum added. The means and standard errors of the means for two experiments with duplicate determinations are shown.
These data suggest that impaired binding of IgG to FcR is a major reason for the reduced ability of rEndoS-treated IgG to support opsonophagocytic killing of the bacteria. In addition, the loss of classical pathway-activating properties may be expected to severely limit protective functions of the antibodies. Taken together, the data emphasize the role of N-linked oligosaccharides on IgG in opsonization and killing of S. pyogenes in blood.
SpeB-mediated cleavage of purified opsonizing human antibodies results in enhanced growth of S. pyogenes in human blood.
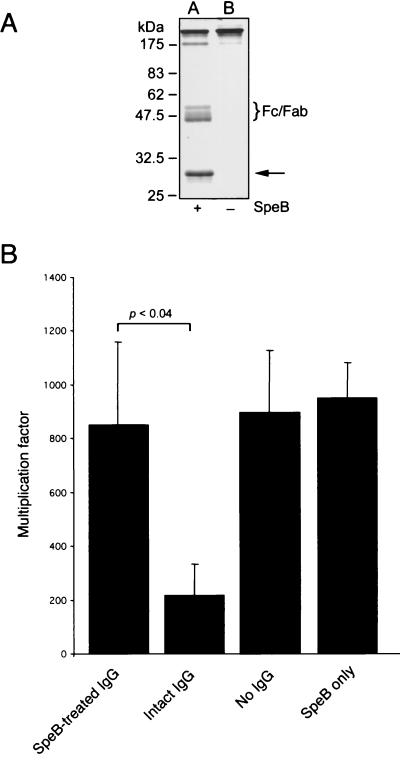
SpeB, an abundant extracellular protein from S. pyogenes, can cleave IgG in a papain-like manner into Fc and monomeric Fab fragments (6). Thus, a preparation of IgGops was incubated with SpeB and analyzed in the opsonophagocytic killing assay as described above. To obtain the highest activity of the enzyme without altering the structure of IgG and its disulfide bonds, the concentration of the reducing agent, DTT, was adjusted to 0.2 mM by titration. Reducing conditions are necessary for activation of SpeB (21). The cleavage of IgG was analyzed by SDS-PAGE under nonreducing conditions. This analysis revealed that SpeB partially cleaved the γ-chains, generating 45- to 55-kDa fragments (Fig. 5A). In contrast, when IgG was incubated with DTT alone, no dissociation of the IgG molecule was detected (Fig. 5A). This suggested that no breakage of the disulfide bonds occurred. The variation in the sizes of the fragments generated was due to the heterogeneity of human IgG. Furthermore, the partial cleavage could be explained by the fact that the IgG2 subclass is relatively resistant to proteolysis by papain (13). This is also true for SpeB; IgG2 is significantly more resistant to proteolysis by this enzyme than the other IgG subclasses are (data not shown).
FIG. 5.
SpeB-mediated cleavage of purified human IgGops enhances bacterial growth in blood. (A) Separation of IgG incubated with (+) or without (−) SpeB by nonreducing SDS-PAGE. Lane A, IgG incubated with SpeB and DTT; lane B, IgG incubated with DTT alone. The brace indicates the position of the IgG Fc and monomeric Fab fragments generated. The arrow indicates the position of the added SpeB protein. (B) Representative results of opsonophagocytic killing assays performed with SpeB-treated IgG and S. pyogenes strain AP1. IgG treated with either SpeB (SpeB-treated IgG) or DTT alone (intact IgG) was added to fresh heparinized human blood. No IgG and SpeB alone were used as controls. Growth of bacteria was monitored after 3 h of growth, and the multiplication factors were calculated. The mean multiplication factors (n = 5) and standard deviations are shown.
SpeB-treated IgGops was subsequently used in the opsonophagocytic killing assay to monitor bacterial survival in blood. This analysis showed that SpeB-treated IgGops, but not untreated IgGops, was significantly impaired in the ability to promote killing of the bacteria (Fig. 5B). The bacteria multiplied in whole blood containing SpeB-treated IgGops, as well as in blood to which no IgG was added (Fig. 5B). Addition of active SpeB alone to the opsonophagocytic killing assay mixture in the absence of IgGops resulted in bacterial multiplication comparable to that obtained when no IgG was added (Fig. 5B). This suggests that SpeB acts on IgG and neutralizes the opsonizing capacity by cleaving IgG into Fc and monomeric Fab fragments, resulting in increased bacterial survival.
DISCUSSION
The host relies on opsonizing antibodies to trigger protective functions of the immune system. This study revealed two possible strategies that the human pathogen S. pyogenes may use to allow blood-borne bacteria to overcome the effects of opsonizing antibodies produced by the host. One strategy is represented by the activity of extracellular SpeB. This enzyme severs the IgG molecule in the hinge region. Thus, the antigen-recognizing Fab portion is separated from the effector-triggering Fc portion, resulting in a damaged IgG molecule. SpeB is the major protein secreted from S. pyogenes, and its role during infection has been shown in several in vivo models (22, 39). Interestingly, SpeB can cleave IgG in the hinge region when bacteria are grown in human plasma (6), suggesting that this also might occur during an infection.
Further support for a strategy in which streptococci attack immunoglobulins was recently obtained when a second secreted cysteine proteinase from the S. pyogenes AP1 strain was identified (41). This IgG-degrading enzyme of S. pyogenes, IdeS, specifically cleaves the hinge region of human IgG and IgG subclasses but not other immunoglobulin isotypes. Interestingly, IdeS cleaves IgG at the same position as SpeB, and in vitro experiments revealed that the IdeS activity on opsonizing antibodies inhibits killing of S. pyogenes by phagocytic cells (41). A second strategy involves activity of EndoS that specifically hydrolyzes the conserved N-linked oligosaccharides on IgG. Removal of these oligosaccharides, as suggested by Radaev and Sun (28), most likely changes the structure of the Fc portion from an open conformation to a more closed conformation. This study showed that deglycosylated IgG had a 10- to 15-fold-lower affinity for FcR III than intact IgG had. Thus, EndoS-induced alterations in IgG structure may explain the reduced binding to cells expressing FcR.
EndoS cleaves between two N-acetylglucosamine (GlcNAc) molecules in the chitibiose core of IgG (6). This cleavage leaves a single GlcNAc with a fucose attached. Completely deglycosylated IgG binds significantly less efficiently to FcR than IgG containing, for example, the trimannose group (24). Thus, EndoS-mediated cleavage between the two innermost GlcNAc molecules results in deceased FcR binding. This suggests that there is an efficient bacterial strategy to decrease the effector function of IgG.
N-linked oligosaccharides did not appear to be important for the capacity of IgG to activate the alternative complement pathway. On the other hand, it is quite possible that specific opsonizing antibodies do not activate the alternative pathway on the surface of S. pyogenes. Judging from the present data, the MBL pathway does not play any role in IgG-mediated opsonophagocytic killing of the organism (Fig. 4). The functionality of IgG Fc glycosylation has been controversial. A human pathogen that is able to express a highly specific endoglycosidase which results in increased survival in human blood suggests that there is an adaptive feature with which the pathogen modulates a critical host defense molecule. In addition, our study highlights and strengthens the importance of the oligosaccharides in Fc-mediated killing of a microorganism.
In conclusion, we identified two different strategies by which an important human pathogen might interfere with the adaptive immune response. The first strategy is expression of a proteinase that cleaves IgG in the hinge region, and the second involves an IgG-oligosaccharide hydrolase, EndoS. These two activities promote bacterial survival in human blood containing opsonizing antibodies ex vivo. These findings expand our understanding of S. pyogenes pathogenesis and reveal new insights into how this human pathogen might evade immune recognition during infection.
Acknowledgments
Ulla Johannesson is acknowledged for excellent technical assistance.
Grants from the Swedish Research Council (projects 9926 and 14727), the Foundations of Bergvall, Crafoord, Kock, Nilson, Royal Physiographic Society, and Österlund, and the Medical Faculty of Lund University supported this work. This study was conducted within the framework of the QLG1-CT&-2001-01039 project.
Editor: J. T. Barbieri
REFERENCES
- 1.Åkesson, P., J. Cooney, F. Kishimoto, and L. Björck. 1990. Protein H—a novel IgG binding bacterial protein. Mol. Immunol. 27:523-531. [DOI] [PubMed] [Google Scholar]
- 2.Åkesson, P., K.-H. Schmidt, J. Cooney, and L. Björck. 1994. M1 protein and protein H: IgGFc- and albumin-binding streptococcal surface proteins encoded by adjacent genes. Biochem. J. 300:877-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berge, A., and L. Björck. 1995. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J. Biol. Chem. 270:9862-9867. [DOI] [PubMed] [Google Scholar]
- 4.Chuang, P. D., and S. L. Morrison. 1997. Elimination of N-linked glycosylation sites from the human IgA1 constant region: effects on structure and function. J. Immunol. 158:724-732. [PubMed] [Google Scholar]
- 5.Collin, M., and A. Olsén. 2001. Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins. Infect. Immun. 69:7187-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collin, M., and A. Olsén. 2001. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 20:3046-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collin, M., and A. Olsén. 2000. Generation of a mature streptococcal cysteine proteinase is dependent on cell wall-anchored M1 protein. Mol. Microbiol. 36:1306-1318. [DOI] [PubMed] [Google Scholar]
- 8.Cooper, N. R. 1988. Laboratory investigation of complement proteins and complement receptors. Baillers Immunol. Allergy 2:263-293. [Google Scholar]
- 9.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deisenhofer, J. 1981. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry 20:2361-2370. [PubMed] [Google Scholar]
- 11.Dube, R., G. A. Rook, J. Steele, R. Brealey, R. Dwek, T. Rademacher, and J. Lennard-Jones. 1990. Agalactosyl IgG in inflammatory bowel disease: correlation with C-reactive protein. Gut 31:431-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredlund, H., A. G. Sjöholm, B. Selander, E. Holmström, P. Olcén, and D. Danielsson. 1993. Serum bactericidal activity and induction of chemiluminescence of polymorphonuclear leukocytes: complement activation pathway requirements in defense against Neisseria meningitidis. Int. Arch. Allergy Immunol. 100:135-143. [DOI] [PubMed] [Google Scholar]
- 13.Gergely, J., H. H. Fudenberg, and E. van Loghem. 1970. The papain susceptibility of IgG myeloma proteins of different heavy chain subclasses. Immunochemistry 7:1-6. [DOI] [PubMed] [Google Scholar]
- 14.Holder, I. A., and R. Wheeler. 1984. Experimental studies of the pathogenesis of infections owing to Pseudomonas aeruginosa: elastase, an IgG protease. Can. J. Microbiol. 30:1118-1124. [DOI] [PubMed] [Google Scholar]
- 15.Jansen, H. J., D. Grenier, and J. S. Van der Hoeven. 1995. Characterization of immunoglobulin G-degrading proteases of Prevotella intermedia and Prevotella nigrescens. Oral Microbiol. Immunol. 10:138-145. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, U., L. Truedsson, and B. Gustavii. 1983. Complement components in 100 newborns and their mothers determined by electroimmunoassay. Acta Pathol. Microbiol. Immunol. Scand. 91:147-150. [PubMed] [Google Scholar]
- 17.Kagawa, T. F., J. C. Cooney, H. M. Baker, S. McSweeney, M. Liu, S. Gubba, J. M. Musser, and E. N. Baker. 2000. Crystal structure of the zymogen form of the group A Streptococcus virulence factor SpeB: an integrin-binding cysteine protease. Proc. Natl. Acad. Sci. USA 97:2235-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilian, M., J. Mestecky, and R. E. Schrohenloher. 1979. Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect. Immun. 26:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilian, M., and M. W. Russell. 1999. Microbial evasion of IgA function, p. 241-251. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, and J. R. McGhee (ed.), Mucosal immunology. Academic Press, San Diego, Calif.
- 20.Lancefield, R. C. 1948. Differentiation of group A streptococci with a common R antigen into three serological types with special reference to the bactericidal test. J. Exp. Med. 106:525-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, T.-Y., N. P. Neumann, S. D. Elliott, S. Moore, and W. H. Stein. 1963. Chemical properties of streptococcal proteinase and its zymogen. J. Biol. Chem. 238:251-256. [PubMed] [Google Scholar]
- 22.Lukomski, S., S. Sreevatsan, A. Amberg, W. Reichardt, M. Woischnik, A. Podbielski, and J. M. Musser. 1997. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J. Clin. Investig. 99:2574-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Male, C. J. 1979. Immunoglobulin A1 protease production by Haemophilus influenzae and Streptococcus pneumoniae. Infect. Immun. 26:254-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mimura, Y., P. Sondermann, R. Ghirlando, J. Lund, S. P. Young, M. Goodall, and R. Jefferis. 2001. Role of oligosaccharide residues of IgG1-Fc in FcγRIIb binding. J. Biol. Chem. 276:45539-45547. [DOI] [PubMed] [Google Scholar]
- 25.Nesbitt, S. A., and M. A. Horton. 1992. A nonradioactive biochemical characterization of membrane proteins using enhanced chemiluminescence. Anal. Biochem. 206:267-272. [DOI] [PubMed] [Google Scholar]
- 26.Petersen, S. V., S. Thiel, and J. C. Jensenius. 2001. The mannan-binding lectin pathway of complement activation: biology and disease association. Mol. Immunol. 38:133-149. [DOI] [PubMed] [Google Scholar]
- 27.Plaut, A. G., J. V. Gilbert, M. S. Artenstein, and J. D. Capra. 1975. Neisseria gonorrhoeae and Neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science 190:1103-1105. [DOI] [PubMed] [Google Scholar]
- 28.Radaev, S., and P. D. Sun. 2001. Recognition of IgG by Fcγ receptor: the role of Fc glycosylation and the binding of peptide inhibitors. J. Biol. Chem. 276:16478-16483. [DOI] [PubMed] [Google Scholar]
- 29.Rademacher, T. W., S. W. Homans, D. L. Fernandes, R. A. Dwek, T. Mizuochi, T. Taniguchi, and A. Kobata. 1983. Structural and conformational analysis of immunoglobulin-derived N-linked oligosaccharides. Biochem. Soc. Trans. 11:132-134. [PubMed] [Google Scholar]
- 30.Rahman, M. A. A., and D. A. Isenberg. 1996. Glycosylation of IgG in rheumatic disease, p. 101-118. In D. A. Isenberg and T. W. Rademacher (ed.), Abnormalities of IgG glycosylation and immunological disorders. John Wiley & Sons Ltd., Chichester, United Kingdom.
- 31.Rudd, P. M., T. Elliott, P. Cresswell, I. A. Wilson, and R. A. Dwek. 2001. Glycosylation and the immune system. Science 291:2370-2376. [DOI] [PubMed] [Google Scholar]
- 32.Shin, M. H., H. Kita, H. Y. Park, and J. Y. Seoh. 2001. Cysteine protease secreted by Paragonimus westermani attenuates effector functions of human eosinophils stimulated with immunoglobulin G. Infect. Immun. 69:1599-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sjöholm, A. G., B. Selander, S. Östenson, E. Holmström, and C. Soderström. 1991. Normal human serum depleted of C1q, factor D and properdin: its use in studies of complement activation. APMIS 99:1120-1128. [DOI] [PubMed] [Google Scholar]
- 34.Sjöholm, A. G., L. Truedsson, and J. C. Jensenius. 2001. Complement pathways and meningococcal disease: diagnostic aspects, p. 529-547. In A. J. Pollard and M. C. J. Maiden (ed.), Methods in molecular medicine, vol. 67. Human Press Inc., Totowa, N.J. [DOI] [PubMed]
- 35.Steffensen, R., S. Thiel, K. Varming, C. Jersild, and J. C. Jensenius. 2000. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J. Immunol. Methods 241:33-42. [DOI] [PubMed] [Google Scholar]
- 36.Sutton, B. J., and D. C. Phillips. 1983. The three-dimensional structure of the carbohydrate within the Fc fragment of immunoglobulin G. Biochem. Soc. Trans. 11:130-132. [PubMed] [Google Scholar]
- 37.Tack, B. D., and J. W. Prahl. 1976. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry 15:4513-4521. [DOI] [PubMed] [Google Scholar]
- 38.Tao, M. H., and S. L. Morrison. 1989. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J. Immunol. 143:2595-2601. [PubMed] [Google Scholar]
- 39.Tsai, P.-J., C.-F. Kuo, K.-Y. Lin, Y.-S. Lin, H.-Y. Lei, F.-F. Chen, J.-R. Wang, and J.-J. Wu. 1998. Effect of group A streptococcal cysteine proteinase on invasion of epithelial cells. Infect. Immun. 66:1460-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volanakis, J. E. 1998. Overview of the complement system, p. 9-32. In J. E. Volanakis and M. M. Frank (ed.), The human complement system in health and disease. Marcel Dekker Inc., New York, N.Y.
- 41.von Pawel-Rammingen, U., B. P. Johansson, and L. Björck. 2002. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 21:1607-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vorup-Jensen, T., S. V. Petersen, A. G. Hansen, K. Poulsen, W. Schwaeble, R. B. Sim, K. B. Reid, S. J. Davis, S. Thiel, and J. C. Jensenius. 2000. Distinct pathways of mannan-binding lectin (MBL)- and C1-complex autoactivation revealed by reconstitution of MBL with recombinant MBL-associated serine protease-2. J. Immunol. 165:2093-2100. [DOI] [PubMed] [Google Scholar]
- 43.Wright, A., and S. L. Morrison. 1998. Effect of C2-associated carbohydrate structure on Ig effector function: studies with chimeric mouse-human IgG1 antibodies in glycosylation mutants of Chinese hamster ovary cells. J. Immunol. 160:3393-3402. [PubMed] [Google Scholar]