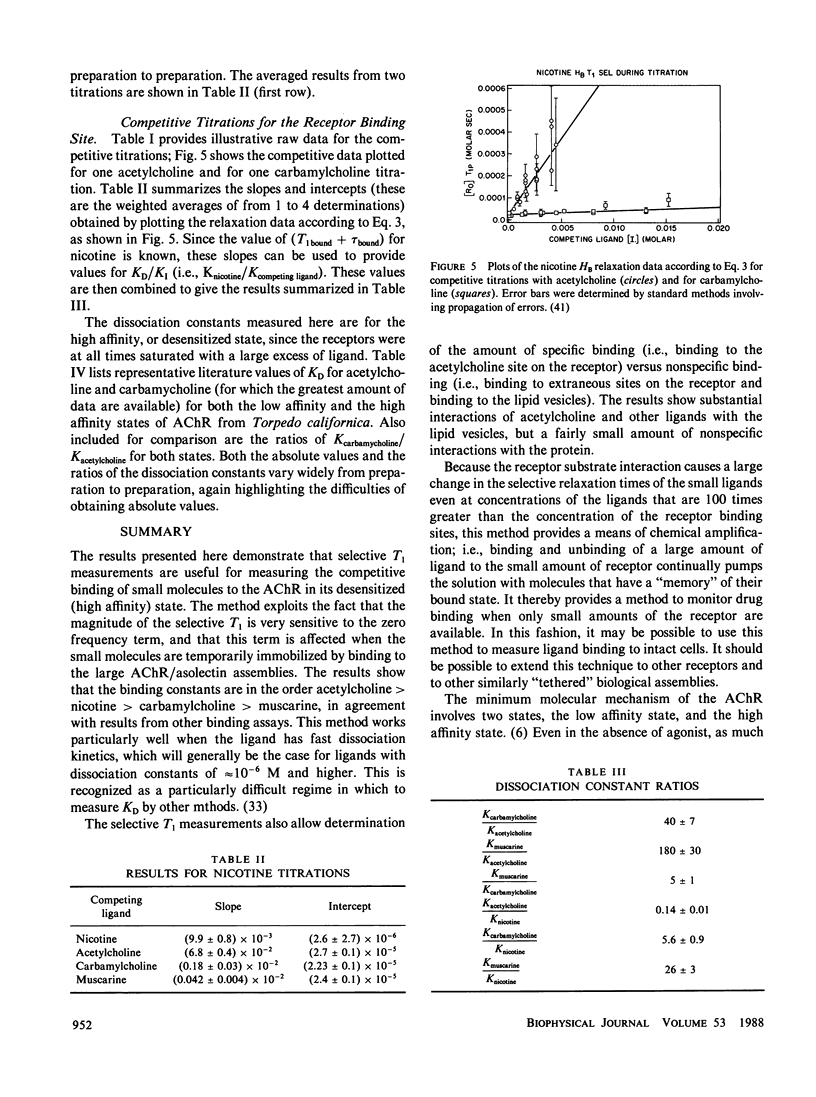
Abstract
A method is presented that uses selective proton Nuclear Magnetic Resonance (NMR) relaxation measurements of nicotine in the presence of the acetylcholine receptor to obtain relative binding constants for acetylcholine, carbamylcholine, and muscarine. For receptors from Torpedo californica the results show that (a) the binding constants are in the order acetylcholine greater than nicotine greater than carbamylcholine greater than muscarine; (b) selective NMR measurements provide a rapid and direct method for monitoring both the specific and nonspecific binding of agonists to these receptors and to the lipid; (c) alpha-bungarotoxin can be used to distinguish between specific and nonspecific binding to the receptor; (d) the receptor--substrate interaction causes a large change in the selective relaxation time of the agonists even at concentrations 100x greater than that of the receptor. This last observation means that these measurements provide a rapid method to monitor drug binding when only small amounts of receptor are available. Furthermore, the binding strategies presented here may be useful for the NMR determination of the conformation of the ligand in its bound state.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd N. D., Cohen J. B. Kinetics of binding of [3H]acetylcholine and [3H]carbamoylcholine to Torpedo postsynaptic membranes: slow conformational transitions of the cholinergic receptor. Biochemistry. 1980 Nov 11;19(23):5344–5353. doi: 10.1021/bi00564a031. [DOI] [PubMed] [Google Scholar]
- Boyd N. D., Cohen J. B. Kinetics of binding of [3H]acetylcholine to Torpedo postsynaptic membranes: association and dissociation rate constants by rapid mixing and ultrafiltration. Biochemistry. 1980 Nov 11;19(23):5353–5358. doi: 10.1021/bi00564a032. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Raftery M. A. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- Costello M. J., Fetter R., Höchli M. Simple procedures for evaluating the cryofixation of biological samples. J Microsc. 1982 Feb;125(Pt 2):125–136. doi: 10.1111/j.1365-2818.1982.tb00330.x. [DOI] [PubMed] [Google Scholar]
- Criado M., Sarin V., Fox J. L., Lindstrom J. Evidence that the acetylcholine binding site is not formed by the sequence alpha 127-143 of the acetylcholine receptor. Biochemistry. 1986 May 20;25(10):2839–2846. doi: 10.1021/bi00358a015. [DOI] [PubMed] [Google Scholar]
- Damle V. N., McLaughlin M., Karlin A. Bromoacetylcholine as an affinity label of the acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1978 Oct 30;84(4):845–851. doi: 10.1016/0006-291x(78)91661-3. [DOI] [PubMed] [Google Scholar]
- Dunn S. M., Blanchard S. G., Raftery M. A. Kinetics of carbamylcholine binding to membrane-bound acetylcholine receptor monitored by fluorescence changes of a covalently bound probe. Biochemistry. 1980 Nov 25;19(24):5645–5652. doi: 10.1021/bi00565a029. [DOI] [PubMed] [Google Scholar]
- Dunn S. M., Raftery M. A. Activation and desensitization of Torpedo acetylcholine receptor: evidence for separate binding sites. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6757–6761. doi: 10.1073/pnas.79.22.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. M., Raftery M. A. Multiple binding sites for agonists on Torpedo californica acetylcholine receptor. Biochemistry. 1982 Nov 23;21(24):6264–6272. doi: 10.1021/bi00267a035. [DOI] [PubMed] [Google Scholar]
- Fong T. M., McNamee M. G. Correlation between acetylcholine receptor function and structural properties of membranes. Biochemistry. 1986 Feb 25;25(4):830–840. doi: 10.1021/bi00352a015. [DOI] [PubMed] [Google Scholar]
- Gibson R. E. Ligand interactions with the acetylcholine receptor from Torpedo californica. Extensions of the allosteric model for cooperativity to half-of-site activity. Biochemistry. 1976 Aug 24;15(17):3890–3901. doi: 10.1021/bi00662a037. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Cash D. J., Aoshima H. Acetylcholine receptor-controlled ion translocation: chemical kinetic investigations of the mechanism. Annu Rev Biophys Bioeng. 1983;12:443–473. doi: 10.1146/annurev.bb.12.060183.002303. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Racker E. Properties of proteoliposomes reconstituted with acetylcholine receptor from Torpedo californica. J Biol Chem. 1982 Aug 25;257(16):9372–9378. [PubMed] [Google Scholar]
- Jain M. K. Studies on a reconstituted acetylcholine receptor system: effect of agonists. Arch Biochem Biophys. 1974 Sep;164(1):20–29. doi: 10.1016/0003-9861(74)90003-4. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Anholt R., Einarson B., Engel A., Osame M., Montal M. Purification of acetylcholine receptors, reconstitution into lipid vesicles, and study of agonist-induced cation channel regulation. J Biol Chem. 1980 Sep 10;255(17):8340–8350. [PubMed] [Google Scholar]
- Maelicke A., Fulpius B. W., Klett R. P., Reich E. Acetylcholine receptor. Responses to drug binding. J Biol Chem. 1977 Jul 25;252(14):4811–4830. [PubMed] [Google Scholar]
- Marks M. J., Collins A. C. Characterization of nicotine binding in mouse brain and comparison with the binding of alpha-bungarotoxin and quinuclidinyl benzilate. Mol Pharmacol. 1982 Nov;22(3):554–564. [PubMed] [Google Scholar]
- Miller J., Witzemann V., Quast U., Raftery M. A. Proton magnetic resonance studies of cholinergic ligand binding to the acetylcholine receptor in its membrane environment. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3580–3584. doi: 10.1073/pnas.76.8.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubig R. R., Cohen J. B. Permeability control by cholinergic receptors in Torpedo postsynaptic membranes: agonist dose-response relations measured at second and millisecond times. Biochemistry. 1980 Jun 10;19(12):2770–2779. doi: 10.1021/bi00553a036. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982 Oct 28;299(5886):793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Ochoa E. L., Dalziel A. W., McNamee M. G. Reconstitution of acetylcholine receptor function in lipid vesicles of defined composition. Biochim Biophys Acta. 1983 Jan 5;727(1):151–162. doi: 10.1016/0005-2736(83)90379-6. [DOI] [PubMed] [Google Scholar]
- Popot J. L., Changeux J. P. Nicotinic receptor of acetylcholine: structure of an oligomeric integral membrane protein. Physiol Rev. 1984 Oct;64(4):1162–1239. doi: 10.1152/physrev.1984.64.4.1162. [DOI] [PubMed] [Google Scholar]
- Ratnam M., Nguyen D. L., Rivier J., Sargent P. B., Lindstrom J. Transmembrane topography of nicotinic acetylcholine receptor: immunochemical tests contradict theoretical predictions based on hydrophobicity profiles. Biochemistry. 1986 May 6;25(9):2633–2643. doi: 10.1021/bi00357a052. [DOI] [PubMed] [Google Scholar]
- Ratnam M., Sargent P. B., Sarin V., Fox J. L., Nguyen D. L., Rivier J., Criado M., Lindstrom J. Location of antigenic determinants on primary sequences of subunits of nicotinic acetylcholine receptor by peptide mapping. Biochemistry. 1986 May 6;25(9):2621–2632. doi: 10.1021/bi00357a051. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Walker J. W., Takeyasu K., McNamee M. G. Activation and inactivation kinetics of Torpedo californica acetylcholine receptor in reconstituted membranes. Biochemistry. 1982 Oct 26;21(22):5384–5389. doi: 10.1021/bi00265a001. [DOI] [PubMed] [Google Scholar]
- Weiland G., Georgia B., Lappi S., Chignell C. F., Taylor P. Kinetics of agonist-mediated transitions in state of the cholinergic receptor. J Biol Chem. 1977 Nov 10;252(21):7648–7656. [PubMed] [Google Scholar]