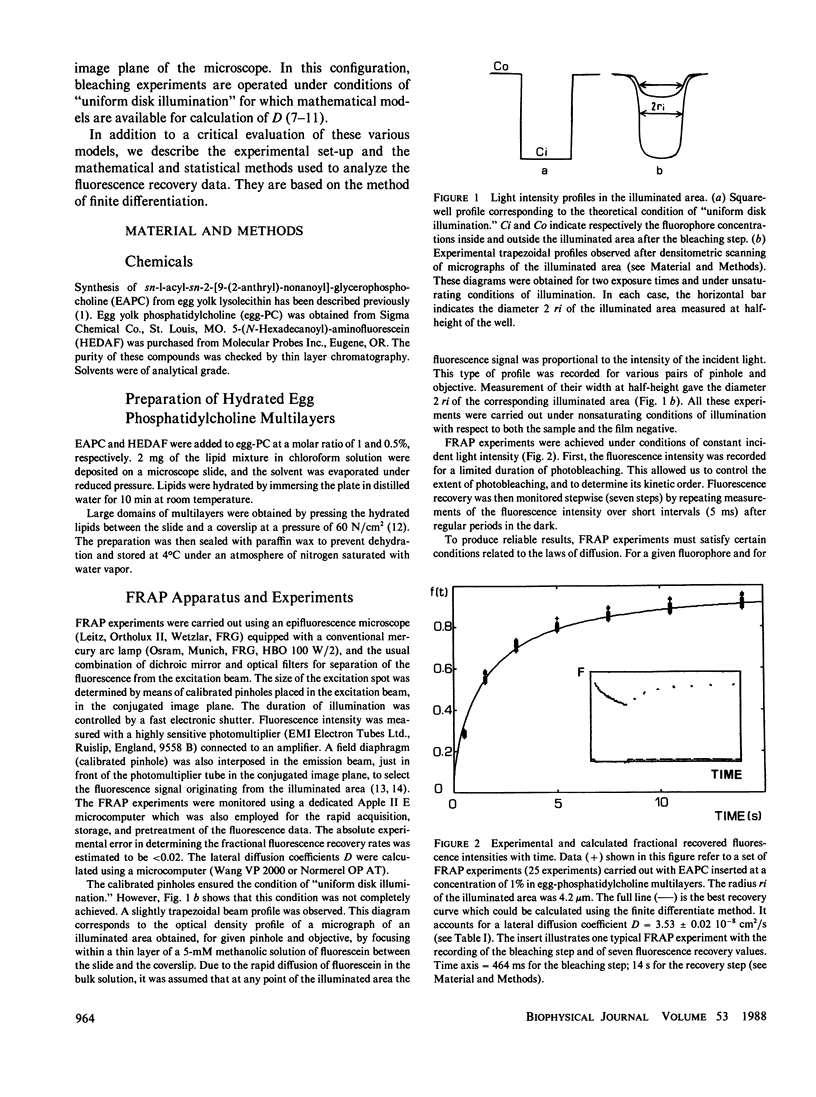
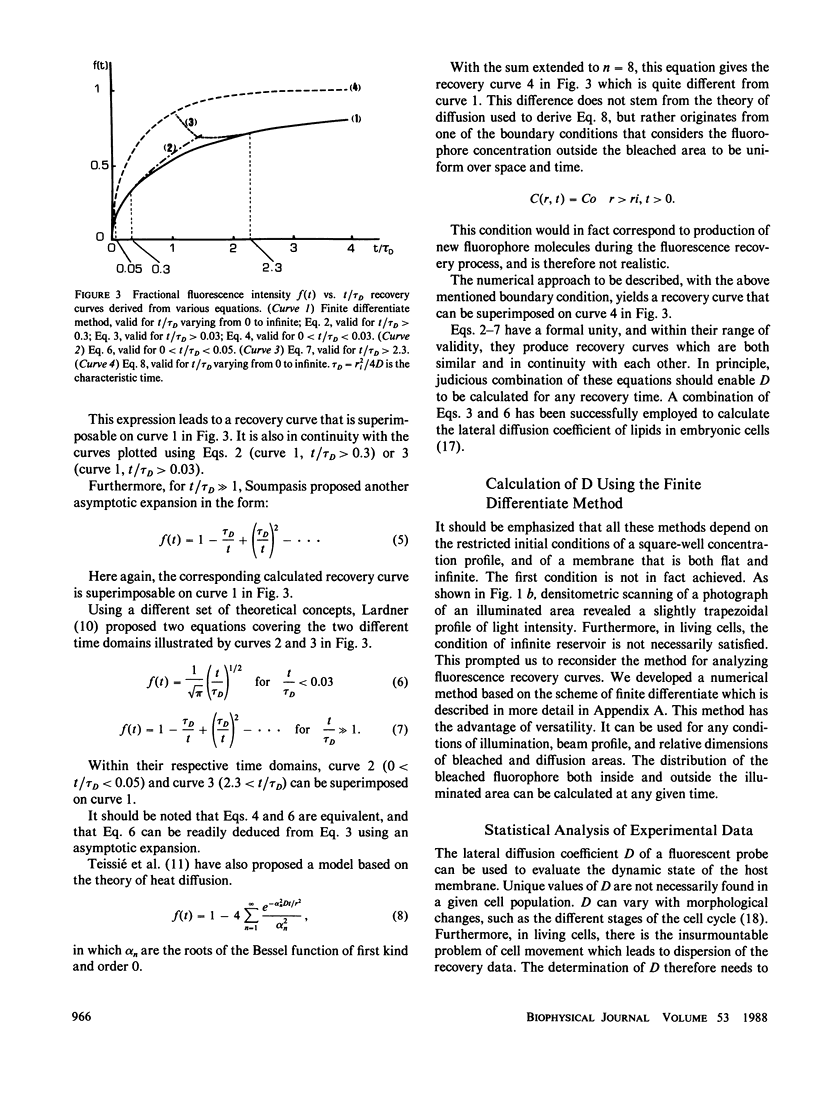
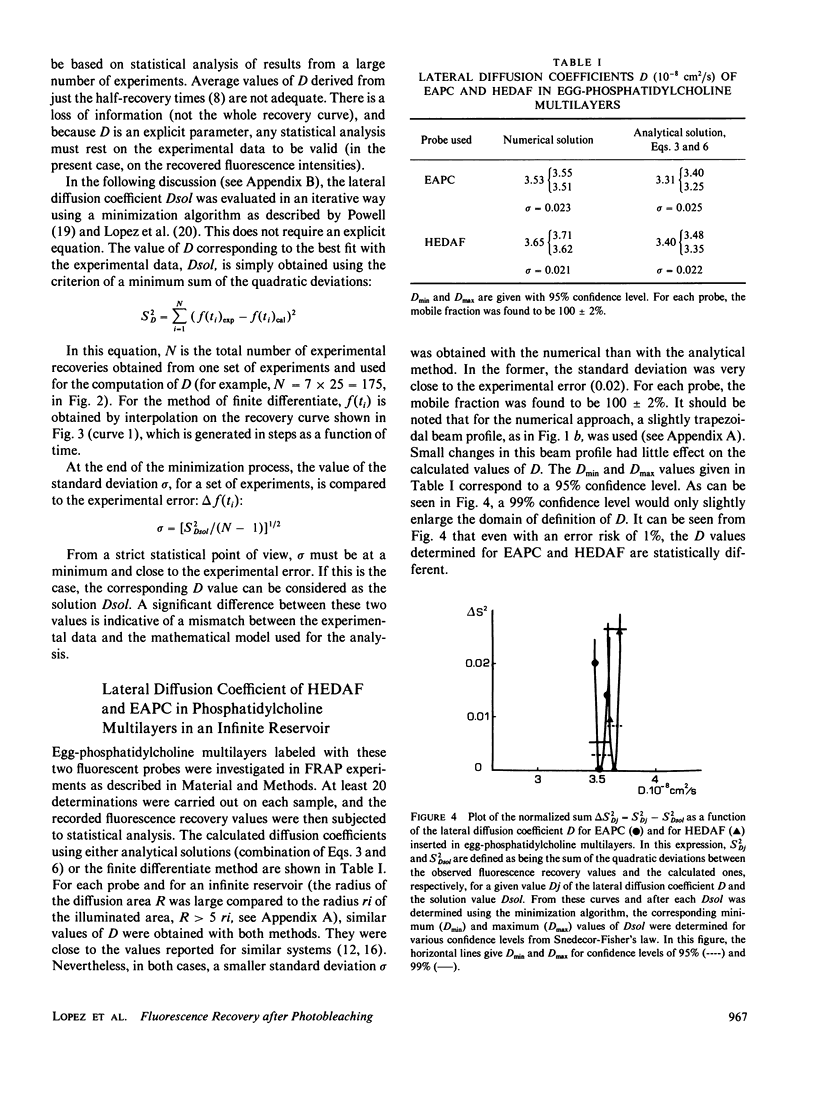
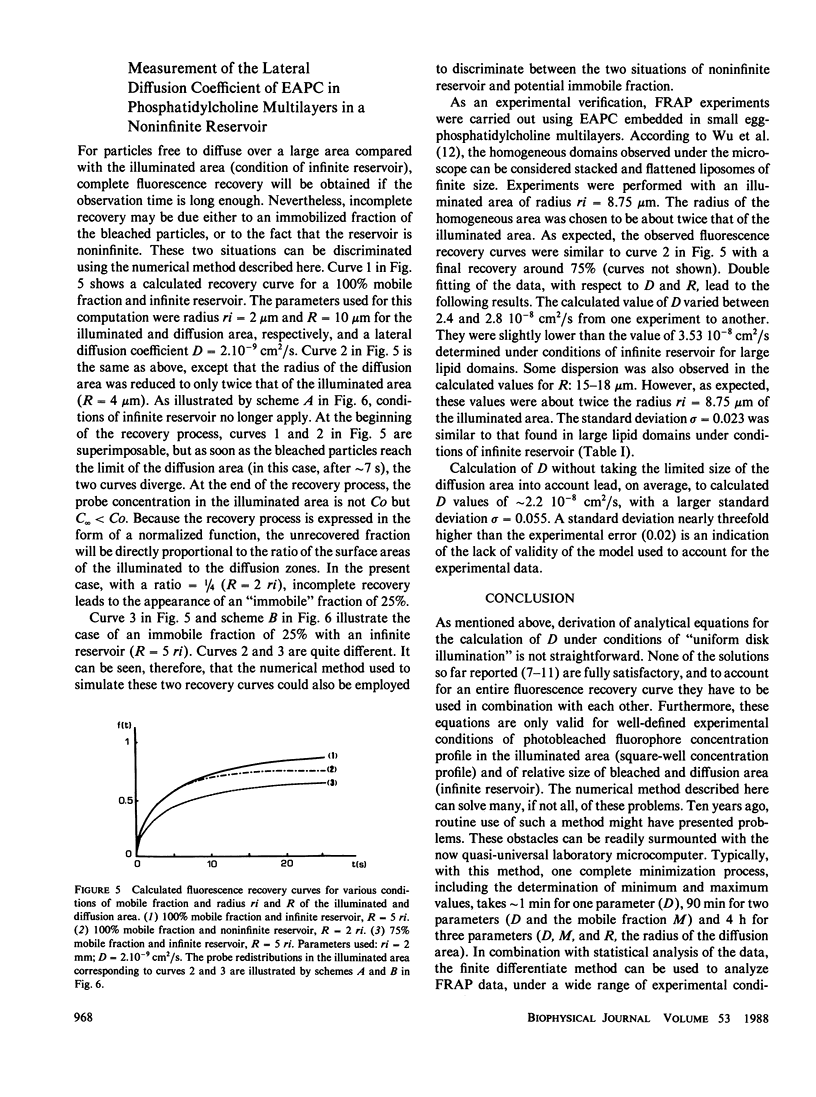
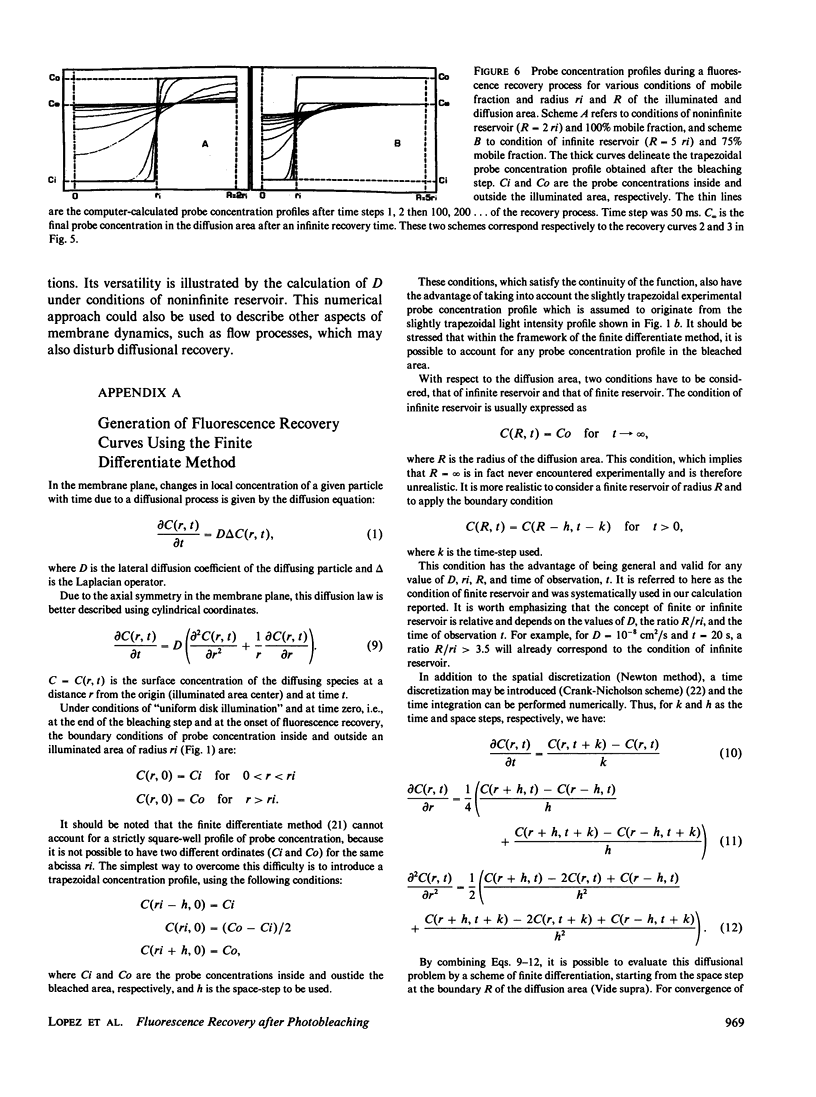
Abstract
A simple fluorescence recovery after photobleaching (FRAP) apparatus using a fluorescence microscope with a conventional mercury arc lamp, working under conditions of "uniform disk illumination" is described. This set-up was designed essentially for the use of anthracene as fluorescent probe, which is bleached (photodimerization reaction) by illumination in the near ultraviolet range (360 nm). It is shown that the lateral diffusion coefficients D can be readily calculated from fluorescence recovery curves using a finite differentiate method in combination with statistical analysis of the data. In contrast to the analytical solutions so far described, this numerical approach is particularly versatile. With a minimization algorithm, D and the probe mobile fraction can be readily calculated for any recovery time under various experimental conditions. These include different probe concentration profiles in the illuminated area after the bleaching step, and situations of infinite or noninfinite reservoir in the diffusion area outside the illuminated area.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupou L., Gualandris L., Lopez A., Duprat A. M., Tocanne J. F. Alterations in lateral lipid mobility in the plasma membrane of urodelean ectodermal cells during gastrulation. Exp Cell Res. 1987 Apr;169(2):502–513. doi: 10.1016/0014-4827(87)90210-2. [DOI] [PubMed] [Google Scholar]
- Dupou L., Teissié J., Tocanne J. F. Metabolic incorporation of 9-(2-anthryl)-nonanoic acid, a new fluorescent and photoactivable probe, into the membrane lipids of Chinese hamster ovary cells. Eur J Biochem. 1986 Jan 2;154(1):171–177. doi: 10.1111/j.1432-1033.1986.tb09375.x. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardner T. J. The measurement of cell membrane diffusion coefficients. J Biomech. 1977;10(3):167–170. doi: 10.1016/0021-9290(77)90055-0. [DOI] [PubMed] [Google Scholar]
- Peters R., Brünger A., Schulten K. Continuous fluorescence microphotolysis: A sensitive method for study of diffusion processes in single cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):962–966. doi: 10.1073/pnas.78.2.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. O., McConnaughey W. B. Effects of multiple membranes on measurements of cell surface dynamics by fluorescence photobleaching. J Supramol Struct Cell Biochem. 1981;17(3):213–221. doi: 10.1002/jsscb.380170303. [DOI] [PubMed] [Google Scholar]
- Soumpasis D. M. Theoretical analysis of fluorescence photobleaching recovery experiments. Biophys J. 1983 Jan;41(1):95–97. doi: 10.1016/S0006-3495(83)84410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissie J., Tocanne J. F., Baudras A. A fluorescence approach of the determination of translational diffusion coefficients of lipids in phospholipid monolayer at the air-water interface. Eur J Biochem. 1978 Feb 1;83(1):77–85. doi: 10.1111/j.1432-1033.1978.tb12070.x. [DOI] [PubMed] [Google Scholar]
- Vincent M., Gallay J., de Bony J., Tocanne J. F. Steady-state and time-resolved fluorescence anisotropy study of phospholipid molecular motion in the gel phase using 1-palmitoyl-2-[9-(2-anthryl)-nonanoyl] -sn-glycero-3-phosphocholine as probe. Eur J Biochem. 1985 Jul 15;150(2):341–347. doi: 10.1111/j.1432-1033.1985.tb09026.x. [DOI] [PubMed] [Google Scholar]
- Welby M., Tocanne J. F. Evidence for the incorporation of a fluorescent anthracene fatty acid into the membrane lipids of Micrococcus luteus. Biochim Biophys Acta. 1982 Jul 14;689(1):173–176. doi: 10.1016/0005-2736(82)90203-6. [DOI] [PubMed] [Google Scholar]
- de Bony J., Tocanne J. F. Photo-induced dimerization of anthracene phospholipids for the study of the lateral distribution of lipids in membranes. Eur J Biochem. 1984 Sep 3;143(2):373–379. doi: 10.1111/j.1432-1033.1984.tb08383.x. [DOI] [PubMed] [Google Scholar]
- de Laat S. W., van der Saag P. T., Elson E. L., Schlessinger J. Lateral diffusion of membrane lipids and proteins during the cell cycle of neuroblastoma cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1526–1528. doi: 10.1073/pnas.77.3.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]


