Abstract
Although plasmid DNA (pDNA)-based immunization has proven efficacy, the level of immune responses that is achieved by this route of vaccination is often lower than that induced by traditional vaccines, especially for primates and humans. We report here a simple and potent method to enhance pDNA-based vaccination by using two different plasmids encoding viral or bacterial antigens. This method is based on coadministration of low concentrations of a recently described immunopotentiating, Schiff base-forming drug called tucaresol which has led to significant augmentation of antigen-specific humoral and cellular immune responses. Our data suggest that enhancement of the immune response with tucaresol might provide a powerful tool for the further development of pDNA-based immunization for humans.
Successful plasmid DNA (pDNA) immunization is characterized by induction of an immune response that occurs via in vivo gene transfer into somatic cells (6, 10, 15, 36, 39, 42). The transfer of genetic information can be achieved by means that employ viral or nonviral vectors. Both types have problems that limit their potential application in the vaccination process. Viral vectors may possibly be infectious, integrate and disrupt the DNA of normal cells, or induce antivector immune responses, whereas nonviral pDNA vectors have the advantage of being simple and safe, and generally lack immunogenic components, but are readily degraded in vivo (12). pDNA-based immunization is relatively inefficient and depends, among other things, on the frequency of CpG motifs and the ability of a very small amount of the pDNA administered, or the protein that it encodes, to be taken up by costimulatory antigen-presenting cells, survive degradation in the lysosomes, and generate the antigen of interest (15, 26, 34, 44). Furthermore, the relatively poor immune response induced by pDNA vaccination in primates and humans is a major problem (11).
Several strategies have been used to increase the pDNA delivery rate and to enhance the immune response to encoded gene products of interest. These strategies include modification of the mode of delivery, targeting of the antigens, and coadministration of immunostimulatory genes or DNA sequences (3, 5, 8, 11, 12, 15, 20, 25, 26, 34, 41, 43).
Administration of Schiff-base-forming drugs, such as tucaresol, to animals has been reported to potentiate the immune response (30). In this study we investigated the possibility of enhancing immune responses following pDNA injection by combining this mode of immunization with systemic costimulation provided by tucaresol. We detected significant enhancement of antigen-specific humoral and cellular immune responses. Whereas coadministration of plasmids encoding granulocyte-macrophage colony-stimulating factor (GM-CSF) and gamma interferon (IFN-γ) was able to enhance antigen-specific antibody and T-cell responses, respectively, tucaresol was able to exert both effects simultaneously, with levels of induction comparable to or even better than that of either of these potent cytokines.
MATERIALS AND METHODS
Plasmid construction and testing.
All genes were inserted into the pCDNA3 vector (Invitrogen BV, Groningen, The Netherlands). Genes used in this study included the Epstein-Barr virus (EBV) nuclear antigen 4 (EBNA-4) and mycobacterial heat shock protein 65 (Mhsp65) genes, which were used as antigens, and mouse GM-CSF and IFN-γ, which were chosen as immunostimulatory cytokines. Details about the subcloning and testing of these plasmids have been published elsewhere (3, 5).
Mice.
HLA-A∗0201/Kb transgenic mice (kindly provided by L. Sherman, Scripps Laboratories, San Diego, Calif.) used in this study have been described previously (40). This strain was used to enable the measurement of the cytotoxic T-cell response to a defined T-cell epitope restricted by HLA-A2 (4, 5). The surface expression of HLA-A∗0201/Kb was confirmed by using an HLA-A∗0201-specific fluorescein isothiocyanate-conjugated monoclonal antibody (One Lambda, Canoga Park, Calif.) and assessed by flow cytometry using FACScan (Becton Dickinson & Co., Mountain View, Calif.). ACA (H-2f) mice were purchased from Jackson Laboratory, Bar Harbor, Maine. This strain was used because it was previously used successfully for measurement of the immune response to EBNA-4 induced by DNA immunization (3). These mice were propagated and maintained in our specific-pathogen-free environment in the Microbiology and Tumor Biology Center (MTC) animal house at the Karolinska Institute.
Immunization.
DNA immunization was accomplished by intramuscular (i.m.) immunization. Mice were injected in the regenerating tibialis-anterior muscle according to the work of Davis et al. (9) and others (3, 5, 14) by using 20 μg of pDNA/100 μl of phosphate buffered-saline (PBS)/muscle. Mice received either a control plasmid (P3), a plasmid encoding EBNA-4 plus the control plasmid P3 (E4), a plasmid containing an Mhsp65 gene plus the control plasmid P3 (P3M.65), P3M.65 plus GM-CSF expression plasmids (P3M.65 G), or P3M.65 plus IFN-γ expression plasmids (P3M.65 γ). Plasmids were mixed in equal molar quantities. Mice treated with tucaresol [4-(2-formyl-3-hydroxy-phenoxymethyl) benzoic acid; kindly provided by John Rhodes, Glaxo SmithKline, Stevenage, United Kingdom] were immunized with E4 or P3M.65 plasmids (E4, T and P3M.65, T, respectively). Tucaresol was injected subcutaneously (s.c.) separately from the DNA in the flank opposite the site of DNA injection. Different schedules of tucaresol injection were used, as follows: (i) one single injection of 1 mg of tucaresol/100 μl of PBS at the same time as the DNA injection (experiments in Fig. 1 to 4 and four out of six experiments reported in Table 1) or (ii) daily s.c. injection of tucaresol (200 μg/100 μl of PBS per mouse) for 4 days, beginning 24 h after pDNA immunization (two of the six experiments reported in Table 1). Mice received boosting 2 weeks after priming with the same schedule of plasmid and tucaresol.
FIG. 1.
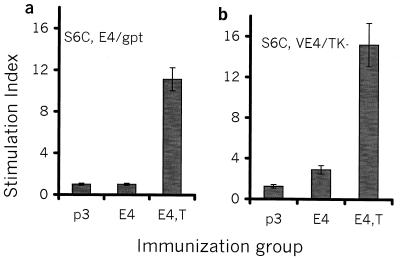
Tucaresol coadministration enhances antigen-specific T-cell proliferative responses. Splenocytes from pDNA-injected mice were cultured in the presence of S6C-E4 cells either alone (a) or infected with V-E4 (S6C-VE4) (b) or in the presence of S6C-gpt control tumor cells either alone (a) or infected with V-TK (S6C-gptV) (b). The SI was calculated as splenocyte proliferation in response to S6C-EBNA-4 transfectants (V-E4 infected) divided by splenocyte proliferation in response to S6C-gptV control transfectants (V-TK infected). Mice were injected twice with 20 μg of pDNA per injection as described in Materials and Methods. Mice from the group treated with tucaresol received 1 mg of tucaresol s.c. simultaneously. The experiment was repeated twice with similar results.
FIG. 4.
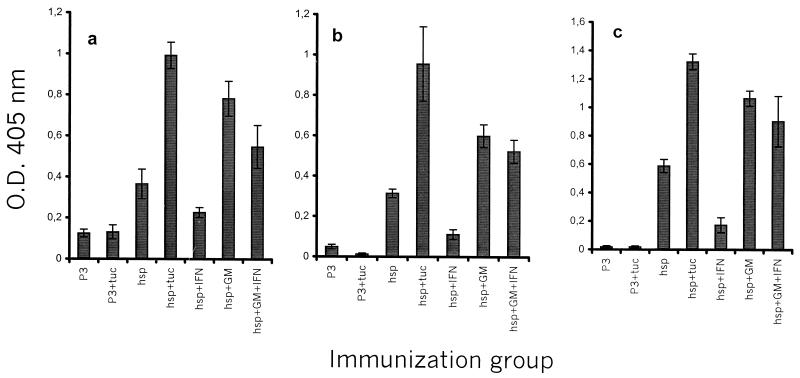
Tucaresol enhances the specific antibody response to Mhsp65 following administration of pDNA encoding Mhsp65. Mice (five per group) were injected twice with 20 μg of pDNA per injection as described in Materials and Methods. Mice from the group treated with tucaresol received 1 mg of tucaresol s.c. simultaneously. Two weeks later, the mice were bled, and antigen-specific IgG (a), IgG2a (b), and IgG1 (c) were detected by ELISA.
TABLE 1.
Effects of tucaresol and cytokine-encoding plasmids on specific antibody responses
| Immunization | IgG
|
IgG2a
|
IgG1
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Responsea |
P relative tob:
|
Response |
P relative to:
|
Response |
P relative to:
|
||||
| P3 | P3M.65 | P3 | P3M.65 | P3 | P3M.65 | ||||
| P3 | 0.23 | 0.05 | 0.08 | ||||||
| P3M.65 | 0.42 | <0.05 | 0.54 | <0.001 | 0.32 | <0.001 | |||
| P3M.65, T | 0.67 | <0.001 | <0.01 | 0.97 | <0.001 | <0.001 | 0.42 | <0.001 | >0.05 |
| P3M.65 G | 0.61 | <0.001 | <0.05 | 0.70 | <0.001 | >0.05 | 0.54 | <0.01 | >0.05 |
| P3M.65 γ | 0.28 | >0.05 | <0.05 | 0.25 | <0.05 | <0.01 | 0.08 | >0.05 | <0.001 |
| P3M.65 G γ | 0.39 | <0.05 | >0.05 | 0.90 | <0.01 | >0.05 | 0.52 | <0.05 | >0.05 |
Each response value is a mean based on the optical densities at 405 nm of individually tested, 1:100-diluted serum samples from six experiments using four to six mice per group. Mice were injected twice with 20 μg of pDNA per injection as described in Materials and Methods. Sera were collected and tested for the presence of Mhsp65-specific antibodies by using isotype-specific ELISA.
Values in each immunization group are compared to those obtained in the P3 and P3M.65 immunization groups. Statistical significance was calculated as P values by using Student's t test.
Cell lines.
Jurkat A∗0201/Kb (Jk-A2/kb) (40), a human T-cell leukemia HLA-A∗0201-negative cell line stably transfected with an HLA-A∗0201/Kb chimeric gene (kindly provided by W. M. Kast, Loyola University, Maywood, Ill.) was used. The S6C cell line was derived from a spontaneous mammary adenocarcinoma that originated in an ACA mouse (21). S6C-gpt and S6C-E4 cells are control plasmid and EBNA-4 transfectants, respectively (38) (kindly provided by George Klein, MTC, Karolinska Institute, Stockholm, Sweden). The S6C-gpt and S6C-E4 cell lines were maintained in vivo by passage in syngeneic ACA mice and in vitro in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 50 μM 2-mercaptoethanol, and 2 mM l-glutamine. Vaccinia virus-infected stimulator cells were prepared by infecting the S6C-E4 and S6C-gpt tumor cell lines with vaccinia viruses expressing the EBNA-4 gene (V-E4) or control vaccinia viruses (V-TK), respectively. Infection was carried out using a multiplicity of infection of 4 for 4 to 6 h. This was followed by treatment of cells with 50 μg of mitomycin C (Sigma, St. Louis, Mo.)/106 cells/ml of PBS for 45 min at 37°C, after which the infected cell lines were washed and then incubated with splenocytes from the immunized mice as described below. Details about vaccinia viruses (kindly provided by M. G. Massuci, MTC, Karolinska Institute) and their use in the infection of these cell lines have been published earlier (27).
T-cell proliferation and cytokine production assay.
Splenocytes harvested from immunized mice were used throughout this study as a source for T cells. Details about the assays have been published elsewhere (3, 5). The proliferation test was performed with triplicate samples, and the stimulation index (SI) was calculated as splenocyte proliferation in response to S6C-EBNA-4 transfectants (V-E4 infected) divided by splenocyte proliferation in response to S6C-gptV control transfectants (V-TK infected). The experiment was repeated twice.
Generation of antigen-specific CTLs and cytotoxicity assays.
Peptide epitope-specific cytotoxic T-lymphocyte (CTL) lines were prepared as described recently (4) by using the Mhsp65 (9.369) 9-mer peptide starting at position 369 (KLAGGVAVI). The influenza virus MP (9.58) 9-mer peptide epitope starting at position 58 (1) was used as a control HLA-A2 binding peptide. The 51Cr release assay was performed as described earlier (5). The experiment was done using pooled splenocytes from four mice per group. The experiment was performed twice.
ELISA procedures.
Sera from injected mice were collected and used in direct enzyme-linked immunosorbent assays (ELISAs) as described earlier (16). Recombinant Mhsp65 was used at a concentration of 4 μg/ml in carbonate buffer to coat the wells of 96-well plates (Maxisorp; Nunc, Roskilde, Denmark) overnight at +4°C. Sera were then added in duplicate at a 1:100 dilution and incubated overnight at +4°C. Binding antibodies were detected by using alkaline phosphatase-conjugated goat antisera specific for mouse immunoglobulin G (IgG) (preabsorbed against mouse IgM), IgG1, and IgG2a (Southern Biotechnology, Birmingham, Ala.).
RESULTS
Tucaresol enhances the specific T-cell proliferative response induced by pDNA injection.
In order to assess the effects of tucaresol in a model system involving T-cell responses, we measured proliferative T-cell responses following i.m. injection with 20 μg of pDNA encoding EBNA-4. Groups of mice were injected i.m. with P3, E4, or E4, T. Following in vitro stimulation with a syngeneic EBNA-4-transfected carcinoma line, S6C-E4, only a minimal proliferative response was detected in splenocyte cultures from E4-immunized mice. Interestingly, a much stronger proliferative response to S6C-E4 cells was obtained among splenocytes from mice injected i.m. with E4 and injected s.c. with tucaresol (Fig. 1a). V-E4-infected stimulator cells (S6C-VE4) also induced a higher proliferative response than S6C-E4 cells from splenocytes of either E4- or E4, T-immunized mice. In contrast, there were no detectable proliferative responses among splenocytes from control (P3)-injected mice against S6C-E4 or S6C-VE4 cells, as assessed by using S6C-gpt or V-TK-infected (S6C-gptV) controls respectively (Fig. 1b). This specific proliferative response, which has been induced by E4, T immunization, was similar to or of a higher magnitude than that obtained from splenocytes of mice immunized with E4 administered in equimolar combination with either a GM-CSF-encoding or an IFN-γ-encoding plasmid (J. Charo, unpublished data).
Tucaresol enhances production of IFN-γ.
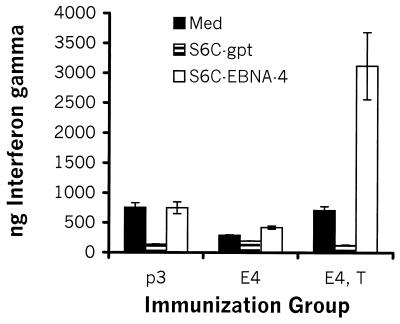
To assess the capacity of tucaresol to enhance a Th1 cytokine response, mice were injected i.m. with either P3, E4, or E4, T. Little, if any, IFN-γ was produced by splenocytes from mice injected with E4 alone following in vitro stimulation with S6C-E4 cells, compared to the levels of IFN-γ produced by control splenocytes from P3-injected mice following in vitro stimulation with S6C-E4 cells (Fig. 2). Interestingly, splenocytes from mice injected i.m. with E4 and s.c. with tucaresol produced the greatest amounts of IFN-γ following in vitro stimulation with S6C-E4 cells but did not do so in response to control stimulation with S6C-gpt cells (Fig. 2). We were unable to detect the Th2 cytokine interleukin-4 (IL-4) in any of the experimental groups (data not shown). The magnitude of the IFN-γ response by splenocytes of E4, T-immunized mice was higher than that obtained from splenocytes of mice immunized with E4 administered in equimolar combination with either a GM-CSF-encoding or an IFN-γ-encoding plasmid (J. Charo, unpublished).
FIG. 2.
Coadministration of tucaresol enhances IFN-γ release. Groups of mice were injected i.m. with P3, E4, or E4, T, and splenocytes were stimulated in vitro with either S6C-E4 tumor cells, S6C-gpt, or medium for 72 h. IFN-γ titers were determined by ELISA. Mice were injected twice with 20 μg of pDNA per injection as described in Materials and Methods. Mice in the group treated with tucaresol received 1 mg of tucaresol s.c. simultaneously. The experiment was repeated twice with similar results.
Augmentation of the specific CTL response by tucaresol.
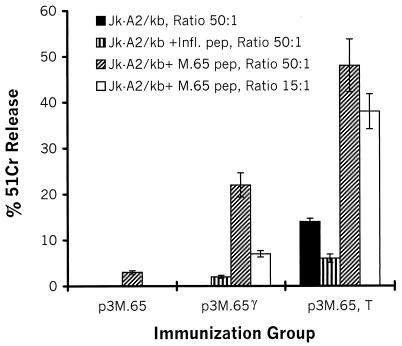
To investigate the effect of tucaresol on the specific CTL response induced by pDNA injection, we immunized HLA-A2 transgenic mice i.m. twice with either P3M.65, P3M.65 in equimolar combination with an IFN-γ-encoding plasmid (P3M.65 γ), or P3M.65 plus 1 mg of tucaresol injected s.c. (P3M.65, T). Two weeks after the last administration, splenocytes from injected mice were stimulated once with an HLA-A2-restricted peptide epitope derived from Mhsp65. Six days later, these in vitro cultures were tested for antigen-specific CTL activity against HLA*A0201/Kb+ Jurkat (Jk-A2/kb) cells either alone or pulsed with either the cognate peptide epitope Mhsp65 (9.369) or a control HLA-A2-restricted influenza virus MP (9.58) peptide epitope. As shown in Fig. 3, splenocytes from mice injected with P3M.65, T developed high CTL activity against target cells pulsed with the cognate Mhsp65 epitope, while their lytic activity against Jk-A2/kb cells alone or pulsed with the control influenza virus peptide epitope was much lower. In contrast, splenocytes from mice immunized with P3M.65 without any costimulatory agent were almost totally inactive (Fig. 3). P3M.65 γ also enhanced the level of CTL activity relative to that of splenocytes derived from mice injected with P3M.65 alone (Fig. 3). However, this population displayed 30 to 50% of the level of cytotoxicity observed with splenocytes from mice that also received tucaresol. We therefore conclude that tucaresol is a very efficient agent that enhances the development of antigen-specific CTLs when it is given together with the appropriate pDNA.
FIG. 3.
CTL responses are markedly enhanced by tucaresol. Groups of HLA-A2/Kb transgenic mice were immunized twice with either P3M.65, P3M.65 γ, or P3M.65, T. Splenocytes were cultured with the HLA-A2 binding peptide Mhsp65 (9.369) for 5 to 6 days and thereafter were used as effectors in conventional 51Cr release assays using as target cells Jk-A2/kb cells pulsed with either Mhsp65 (9.369) (M.65 pep), an irrelevant influenza virus peptide (Infl. pep), or medium, as described in Materials and Methods. Mice were injected twice with 20 μg of pDNA per injection as described in Materials and Methods. Mice in the group treated with tucaresol received 1 mg of tucaresol s.c. simultaneously. The experiment was repeated with similar results.
Tucaresol enhances the production of antigen-specific antibodies induced by pDNA vaccination.
We also analyzed the ability of tucaresol to enhance the antibody response induced by pDNA-based immunization by utilizing a plasmid containing the Mhsp65 gene, and we compared the effect of tucaresol addition to that of coadministration of plasmids encoding GM-CSF or IFN-γ.
Groups of mice were injected i.m. with 20 μg of P3M.65 or with the same amount of a control plasmid (P3). Significant amounts of Mhsp65-specific antibodies could be detected in sera from P3M.65-immunized mice but not in sera from P3-injected mice (Fig. 4a). In addition, antibody titers were significantly increased when 1 mg of tucaresol was coadministered in conjunction with the i.m. injection, and also when tucaresol was administered as four daily injections of 0.2 mg s.c beginning 24 h after injection with P3M.65 (P3M.65, T) (Fig. 4a; Table 1). In contrast, no increase in the antigen-specific antibody response was detected in a group of mice that received the control plasmid and tucaresol (P3, T), thus excluding the possibility that a general increase in nonspecific cross-reactive antibodies, due to the high degree of immunopotentiation associated with tucaresol administration, accounted for the effect observed (Fig. 4a).
We also compared the effect of tucaresol coadministration with that of the P3M.65 plasmid administered either alone or in equimolar combination with either a GM-CSF-encoding plasmid (P3M.65 G), an IFN-γ-encoding plasmid (P3M.65 γ), or a mixture of GM-CSF- and IFN-γ-encoding plasmids (P3M.65 G γ). Mhsp65 antibody titers were increased when the GM-CSF-encoding plasmid was included, with levels comparable to those induced by tucaresol inclusion (Fig. 4a and Table 1). In contrast, the degree of antibody induction observed in mice that also received the IFN-γ plasmid was decreased, suggesting a suppression of antibody production by IFN-γ (Table 1). Combining both cytokine plasmids seemed to antagonize the enhancing effect of the GM-CSF plasmid and resulted in a reduction of the humoral response to levels essentially similar to those observed in mice that received P3M.65 alone (Fig. 4a and Table 1). Collectively, these results demonstrate that tucaresol has the capacity to enhance the antibody response induced by genetic immunization with the Mhsp65 antigen and that this effect is comparable to the effect of the GM-CSF plasmid.
Tucaresol enhances the Th1 antibody response.
To characterize the antibody response in terms of the helper effects that stimulated it, we analyzed the isotypes of the anti-Mhsp65 antibodies that were induced. Although P3M.65-immunized mice produced significantly elevated amounts of Mhsp65-specific IgG2a, these titers were significantly increased (P < 0.001) in mice that received tucaresol as well (Fig. 4b and Table 1). This clear enhancement of a Th1 type antibody response was unique, as immunization with P3M.65 G, P3M.65 γ, or P3M.65 G γ did not exert such an effect (Fig. 4b and Table 1). Inclusion of a plasmid encoding a Th1 cytokine, IFN-γ, in the immunization did not enhance the levels of anti-Mhsp65 IgG2a that were produced. Instead, IFN-γ inclusion resulted in the suppression of both Th1- and Th2-associated antibody responses (Fig. 4b and c; Table 1). Interestingly, a Th2-associated anti-Mhsp65 IgG1 antibody response induced in mice receiving P3M.65 was not suppressed in mice receiving P3M.65, T (Fig. 4c and Table 1). Taken together, our data indicate that a significant increase in the antigen-specific antibody response as a result of genetic immunization could be achieved by coadministration of tucaresol. Not only did the coadministration enhance the Th1-associated antibody response; it also maintained a Th2 type antibody response.
DISCUSSION
We present a simple and very effective approach to enhancement of pDNA-based immunization, by providing a costimulatory signal that acts upon T cells via Schiff base formation. This has been demonstrated in two models for DNA vaccination previously established for induction of specific immune responses against viral (3) and mycobacterial (4, 5) antigens. The immunopotentiating effect of tucaresol has been reported to be mediated by delivery of costimulatory signals involved in the priming of T-cell responses through the substitution of carbonyl groups naturally provided by antigen-presenting cells. This costimulatory function results in a covalent and reversible reaction that works via binding to the amine-rich groups on T cells, which leads to T-cell-receptor-independent signaling (7). The effect of tucaresol is thought to be CD2 mediated (7, 29, 32, 33). Our observations demonstrate that this approach is able to overcome the low efficacy commonly associated with pDNA-based vaccination (11).
It is clear that induction of an adequate immune response requires the participation of multiple components of the immune system and that pDNA-based immunization is capable of fulfilling this requirement, because it can induce both humoral and cellular responses, including CTL responses (22). While other modes of enhancing pDNA immunization have generally led to either an antibody- or a T-cell-biased immune response (15, 43), coinjection of tucaresol resulted in a general enhancement of both, involving enhancement of a Th1-associated antibody response without decreasing the Th2-associated antibody response. Combined administration of pDNA and tucaresol might therefore be considered under conditions where either a cellular or an antibody-based immune response would be beneficial for the host.
Tucaresol has been suggested to have many potentially useful therapeutic applications, including treatment of infectious diseases caused by intracellular pathogens and therapy for certain types of cancers. Several clinical studies have been performed involving injection of tucaresol in healthy volunteers and in sickle cell anemia patients, focusing on its ability to modify hemoglobin, and recently it has also been studied for treatment of other conditions, such as metastatic melanoma (7, 29, 32, 33). Therefore, a therapeutic strategy that combines DNA vaccination and tucaresol administration, e.g., in immunotherapy for melanoma patients, may benefit not only from the indirect adjuvant effect of tucaresol but also from its direct therapeutic effect.
The general utility of this approach is readily apparent and equally efficient when applied to genes of bacterial (Mycobacterium bovis) or viral (EBV) origin. Both M. bovis and EBV genes are derived from pathogens which are involved in infectious and/or neoplastic diseases of considerable importance from both a clinical standpoint and a global health perspective (2, 13, 18, 24), and there is currently an urgent need for prophylactic vaccines against these pathogens. hsp65 is an immunodominant component of both tuberculosis and leprosy (19). Data indicate that Mycobacterium leprae-derived hsp65 can induce prophylactic and therapeutic immunity against experimental tuberculosis (2, 23, 37). Furthermore, EBNA-4-based pDNA vaccination enhanced by tucaresol as a costimulant is of considerable interest in relation to the development of new therapeutic or prophylactic vaccination protocols to be tested clinically. EBV infection is associated with pathological conditions that include infectious mononucleosis and cancer (24). It has recently been reported that pDNA vaccination using a plasmid encoding EBNA-4 could protect mice from the outgrowth of tumors expressing this gene (3).
The marked ability of tucaresol to enhance pDNA-induced specific T-cell responses to hsp65 and EBNA-4, as detected by proliferation, cytokine production, and generation of antigen-specific CTLs, is of particular importance. Protective and therapeutic immunity to mycobacterial infection, EBV infection, and other infectious diseases as well as cancer is dependent on both CD4+ helper cells and cytotoxic CD8+ cells (17, 28, 31, 35). Since pDNA vaccination combined with tucaresol favors both CD4- and CD8-mediated T-cell responses, as shown here, this would be an attractive mode of vaccination to be further explored for new T-cell-targeted vaccines against intracellular bacteria and viruses.
Tucaresol is a chemically well defined molecule that is not overtly toxic and has already been clinically tested. This provides an advantage in the application of this drug in pDNA vaccination protocols. Furthermore, since tucaresol has been shown to be systemically active (30, 33), there may be no need for local coadministration of pDNA and tucaresol, as shown here by combining i.m. pDNA injection with s.c. injection of tucaresol.
Since tucaresol is an orally bioavailable drug (29), the combination of intradermal “ballistic” delivery of pDNA with oral administration of tucaresol may prove to be a very attractive mode of immunization, particularly under conditions where parenteral immunizations should be avoided due to risks of blood-borne infections from contaminated needles or cultural stigmata associated with injections (33, 36). It is therefore noteworthy that tucaresol also significantly enhances the specific immune response induced by gene gun-based pDNA vaccination (J. Charo, J. A. Lindencrona, J. Hinkula, and R. Kiessling, unpublished data).
Production of antigen-specific antibodies was significantly increased by tucaresol coadministration, as indicated by the data presented in Fig. 4 and Table 1 as well as by the observation that the highest IgG/IgM ratio among the experimental groups was achieved by coinjection of tucaresol (data not shown). The induction of a generalized immune response by combining pDNA vaccination with tucaresol administration points out the potential advantage of using this procedure for production of monoclonal antibodies.
In summary, the data presented here show for the first time the utility of using a Schiff-base-forming drug as an adjuvant for induction of both humoral and cellular, antigen-specific immune responses induced by pDNA injection. These data indicate that this strategy may be valuable in the clinical setting for augmenting responses to DNA-based prophylactic and therapeutic vaccines.
Acknowledgments
We thank Maj-Lis Solberg and Margareta Hagelin for excellent technical assistance.
This work was supported by grants from the Swedish Cancer Society, the Cancer Society of Stockholm, the King Gustaf V Jubilee Fund, and the European Community, by a grant awarded to R.K. from the Swedish Gene Therapy Program, and by a grant from GlaxoSmithKline to R.K. J.C. is a recipient of the Karolinska Institute and MTC doctorate award.
Editor: J. D. Clements
REFERENCES
- 1.Bednarek, M. A., S. Y. Sauma, M. C. Gammon, G. Porter, S. Tamhankar, A. R. Williamson, and H. J. Zweerink. 1991. The minimum peptide epitope from the influenza virus matrix protein. Extra and intracellular loading of HLA-A2. J. Immunol. 147:4047-4053. [PubMed] [Google Scholar]
- 2.Bloom, B. R., and J. D. McKinney. 1999. The death and resurrection of tuberculosis. Nat. Med. 5:872-874. [DOI] [PubMed] [Google Scholar]
- 3.Charo, J., A. M. Ciupitu, A. Le Chevalier De Preville, P. Trivedi, G. Klein, J. Hinkula, and R. Kiessling. 1999. A long-term memory obtained by genetic immunization results in full protection from a mammary adenocarcinoma expressing an EBV gene. J. Immunol. 163:5913-5919. [PubMed] [Google Scholar]
- 4.Charo, J., A. Geluk, M. Sundbäck, B. Mirzai, A. D. Diehl, K. J. Malmberg, A. Achour, S. Huriguchi, K. E. van Meijgaarden, J. W. Drijfhout, N. Beekman, P. van Veelen, F. Ossendorp, T. Ottenhoff, and R. Kiessling. 2001. Identification of a common pathogen-specific HLA class I A∗0201 restricted cytotoxic T-cell epitope encoded within the heat shock protein 65. Eur. J. Immunol. 31:3602-3611. [DOI] [PubMed] [Google Scholar]
- 5.Charo, J., M. Sundbäck, A. Geluk, T. Ottenhoff, and R. Kiessling. 2001. DNA immunization of HLA transgenic mice with a plasmid expressing the mycobacterial heat shock protein 65 results in HLA class I- and II-restricted T-cell responses that can be augmented by cytokines. Hum. Gene Ther. 12:1797-1804. [DOI] [PubMed] [Google Scholar]
- 6.Chattergoon, M. A., T. M. Robinson, J. D. Boyer, and D. B. Weiner. 1998. Specific immune induction following DNA-based immunization through in vivo transfection and activation of macrophages/antigen-presenting cells. J. Immunol. 160:5707-5718. [PubMed] [Google Scholar]
- 7.Chen, H., S. Hall, B. Heffernan, N. T. Thompson, M. V. Rogers, and J. Rhodes. 1997. Convergence of Schiff base costimulatory signaling and TCR signaling at the level of mitogen-activated protein kinase ERK2. J. Immunol. 159:2274-2281. [PubMed] [Google Scholar]
- 8.Cohen, A. D., J. D. Boyer, and D. B. Weiner. 1998. Modulating the immune response to genetic immunization. FASEB J. 12:1611-1626. [PubMed] [Google Scholar]
- 9.Davis, H. L., B. A. Demeneix, B. Quantin, J. Coulombe, and R. G. Whalen. 1993. Plasmid DNA is superior to viral vectors for direct gene transfer into adult mouse skeletal muscle. Hum. Gene Ther. 4:733-740. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 11.Ertl, H. C., and Z. Xiang. 1996. Novel vaccine approaches. J. Immunol. 156:3579-3582. [PubMed] [Google Scholar]
- 12.Felgner, P. L. 1997. Nonviral strategies for gene therapy. Sci. Am. 276:102-106. [DOI] [PubMed] [Google Scholar]
- 13.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 14.Fomsgaard, A., H. V. Nielsen, C. Nielsen, K. Johansson, R. Machuca, L. Bruun, J. Hansen, and S. Buus. 1998. Comparisons of DNA-mediated immunization procedures directed against surface glycoproteins of human immunodeficiency virus type-1 and hepatitis B virus. APMIS 106:636-646. [DOI] [PubMed] [Google Scholar]
- 15.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 16.Hajeer, A. H., J. Worthington, K. Morgan, and R. M. Bernstein. 1992. Monoclonal antibody epitopes of mycobacterial 65-kD heat-shock protein defined by epitope scanning. Clin. Exp. Immunol. 89:115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harboe, M., P. Andersen, M. J. Colston, B. Gicquel, P. W. Hermans, J. Ivanyi, and S. H. Kaufmann. 1996. European Commission COST/STD Initiative. Report of the expert panel IX. Vaccines against tuberculosis. Vaccine 14:701-716. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann, S. H. 1997. Antibacterial vaccines: impact of antigen handling and immune response. J. Mol. Med. 75:360-363. [DOI] [PubMed] [Google Scholar]
- 19.Kiessling, R., A. Gronberg, J. Ivanyi, K. Soderstrom, M. Ferm, S. Kleinau, E. Nilsson, and L. Klareskog. 1991. Role of hsp60 during autoimmune and bacterial inflammation. Immunol. Rev. 121:91-111. [DOI] [PubMed] [Google Scholar]
- 20.Krieg, A. M., A. K. Yi, J. Schorr, and H. L. Davis. 1988. The role of CpG dinucleotides in DNA vaccines. Trends Microbiol. 6:23.. [DOI] [PubMed] [Google Scholar]
- 21.Kuzumaki, N., I. A. More, A. J. Cochran, and G. Klein. 1980. Thirteen new mammary tumor cell lines from different mouse strains. Eur. J. Cancer 16:1181-1192. [DOI] [PubMed] [Google Scholar]
- 22.Liu, M. A. 1997. The immunologist's grail: vaccines that generate cellular immunity. Proc. Natl. Acad. Sci. USA 94:10496-10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowrie, D. B., R. E. Tascon, V. L. Bonato, V. M. Lima, L. H. Faccioli, E. Stavropoulos, M. J. Colston, R. G. Hewinson, K. Moelling, and C. L. Silva. 1999. Therapy of tuberculosis in mice by DNA vaccination. Nature 400:269-271. [DOI] [PubMed] [Google Scholar]
- 24.Magrath, I. T., and J. G. Judde. 1997. Epstein-Barr virus and neoplasia, p. 642-651. In J. R. Bertino (ed.), Encyclopedia of cancer, vol. I. Academic Press, San Diego, Calif.
- 25.Manickan, E., S. Kanangat, R. J. Rouse, Z. Yu, and B. T. Rouse. 1997. Enhancement of immune response to naked DNA vaccine by immunization with transfected dendritic cells. J. Leukoc. Biol. 61:125-132. [DOI] [PubMed] [Google Scholar]
- 26.Mir, L. M., M. F. Bureau, J. Gehl, R. Rangara, D. Rouy, J. M. Caillaud, P. Delaere, D. Branellec, B. Schwartz, and D. Scherman. 1999. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl. Acad. Sci. USA 96:4262-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray, R. J., M. G. Kurilla, J. M. Brooks, W. A. Thomas, M. Rowe, E. Kieff, and A. B. Rickinson. 1992. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J. Exp. Med. 176:157-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Reilly, R. J., J. F. Lacerda, K. G. Lucas, N. S. Rosenfield, T. N. Small, and E. B. Papadopoulos. 1996. Adoptive cell therapy with donor lymphocytes for EBV-associated lymphomas developing after allogeneic marrow transplants. Important Adv. Oncol. 1996:149-166. [PubMed] [Google Scholar]
- 29.Rhodes, J. 1996. Covalent chemical events in immune induction: fundamental and therapeutic aspects. Immunol. Today 17:436-441. [DOI] [PubMed] [Google Scholar]
- 30.Rhodes, J., H. Chen, S. R. Hall, J. E. Beesley, D. C. Jenkins, P. Collins, and B. Zheng. 1995. Therapeutic potentiation of the immune system by costimulatory Schiff-base-forming drugs. Nature 377:71-75. [DOI] [PubMed] [Google Scholar]
- 31.Rickinson, A. B., and E. D. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa..
- 32.Rolan, P. E., A. J. Mercer, R. Wootton, and J. Posner. 1995. Pharmacokinetics and pharmacodynamics of tucaresol, an antisickling agent, in healthy volunteers. Br. J. Clin. Pharmacol. 39:375-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rolan, P. E., J. E. Parker, S. J. Gray, B. C. Weatherley, J. Ingram, W. Leavens, R. Wootton, and J. Posner. 1993. The pharmacokinetics, tolerability and pharmacodynamics of tucaresol (589C80; 4[2-formyl-3-hydroxyphenoxymethyl] benzoic acid), a potential anti-sickling agent, following oral administration to healthy subjects. Br. J. Clin. Pharmacol. 35:419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roman, M., E. Martin-Orozco, J. S. Goodman, M. D. Nguyen, Y. Sato, A. Ronaghy, R. S. Kornbluth, D. D. Richman, D. A. Carson, and E. Raz. 1997. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 3:849-854. [DOI] [PubMed] [Google Scholar]
- 35.Rooney, C. M., C. A. Smith, and H. E. Heslop. 1997. Control of virus-induced lymphoproliferation: Epstein-Barr virus-induced lymphoproliferation and host immunity. Mol. Med. Today 3:24-30. [DOI] [PubMed] [Google Scholar]
- 36.Tang, D. C., M. DeVit, and S. A. Johnston. 1992. Genetic immunization is a simple method for eliciting an immune response. Nature 356:152-154. [DOI] [PubMed] [Google Scholar]
- 37.Tascon, R. E., M. J. Colston, S. Ragno, E. Stavropoulos, D. Gregory, and D. B. Lowrie. 1996. Vaccination against tuberculosis by DNA injection. Nat. Med. 2:888-892. [DOI] [PubMed] [Google Scholar]
- 38.Trivedi, P., G. Winberg, and G. Klein. 1997. Differential immunogenicity of Epstein-Barr virus (EBV) encoded growth transformation-associated antigens in a murine model system. Eur. J. Cancer 33:912-917. [DOI] [PubMed] [Google Scholar]
- 39.Ulmer, J. B., J. J. Donnelly, S. E. Parker, G. H. Rhodes, P. L. Felgner, V. J. Dwarki, S. H. Gromkowski, R. R. Deck, C. M. DeWitt, A. Friedman, et al. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745-1749. [DOI] [PubMed] [Google Scholar]
- 40.Vitiello, A., D. Marchesini, J. Furze, L. A. Sherman, and R. W. Chesnut. 1991. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J. Exp. Med. 173:1007-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widera, G., M. Austin, D. Rabussay, C. Goldbeck, S. W. Barnett, M. Chen, L. Leung, G. R. Otten, K. Thudium, M. J. Selby, and J. B. Ulmer. 2000. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 164:4635-4640. [DOI] [PubMed] [Google Scholar]
- 42.Wolff, J. A., R. W. Malone, P. Williams, W. Chong, G. Acsadi, A. Jani, and P. L. Felgner. 1990. Direct gene transfer into mouse muscle in vivo. Science 247:1465-1468. [DOI] [PubMed] [Google Scholar]
- 43.Xiang, Z., and H. C. Ertl. 1995. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity 2:129-135. [DOI] [PubMed] [Google Scholar]
- 44.Xiang, Z. Q., Z. He, Y. Wang, and H. C. Ertl. 1997. The effect of interferon-gamma on genetic immunization. Vaccine 15:896-898. [DOI] [PubMed] [Google Scholar]