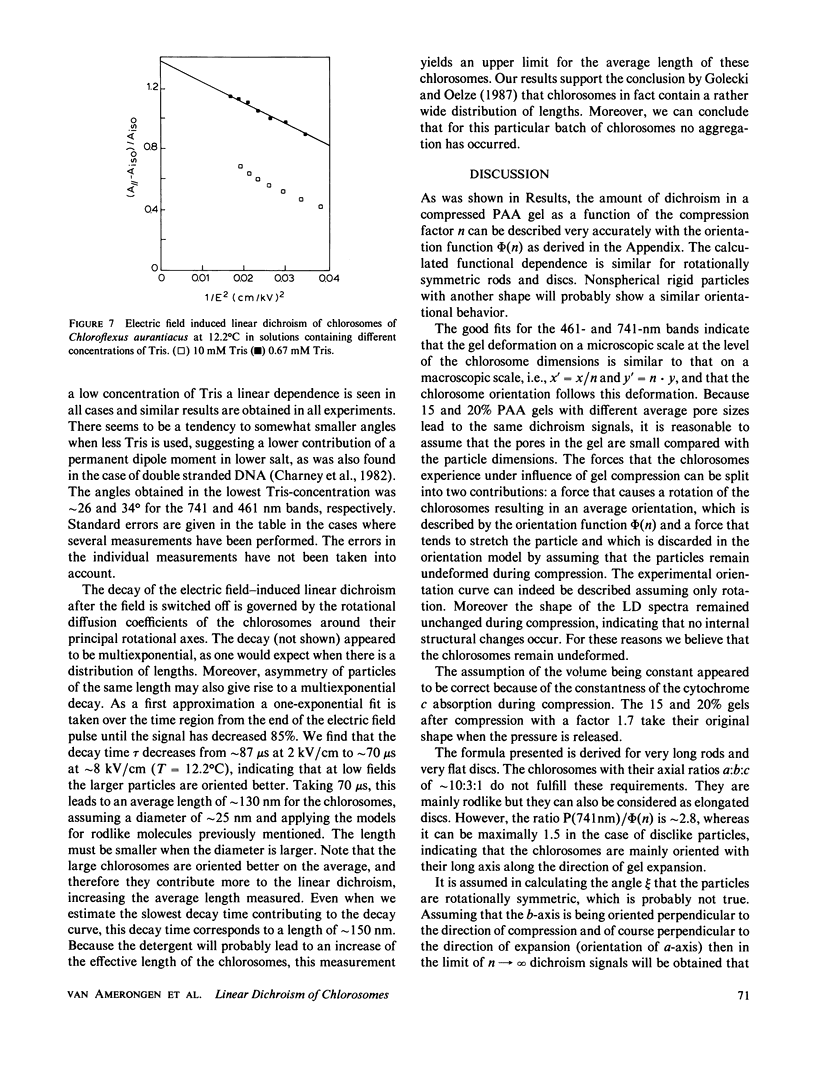
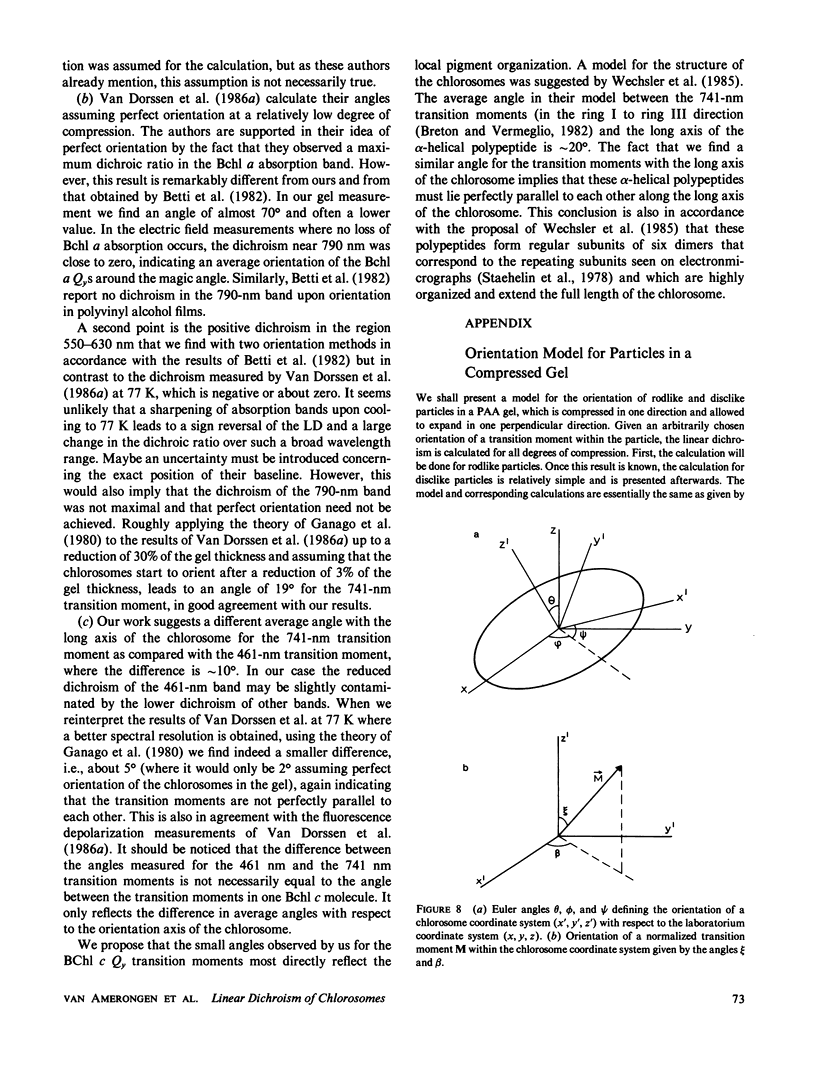
Abstract
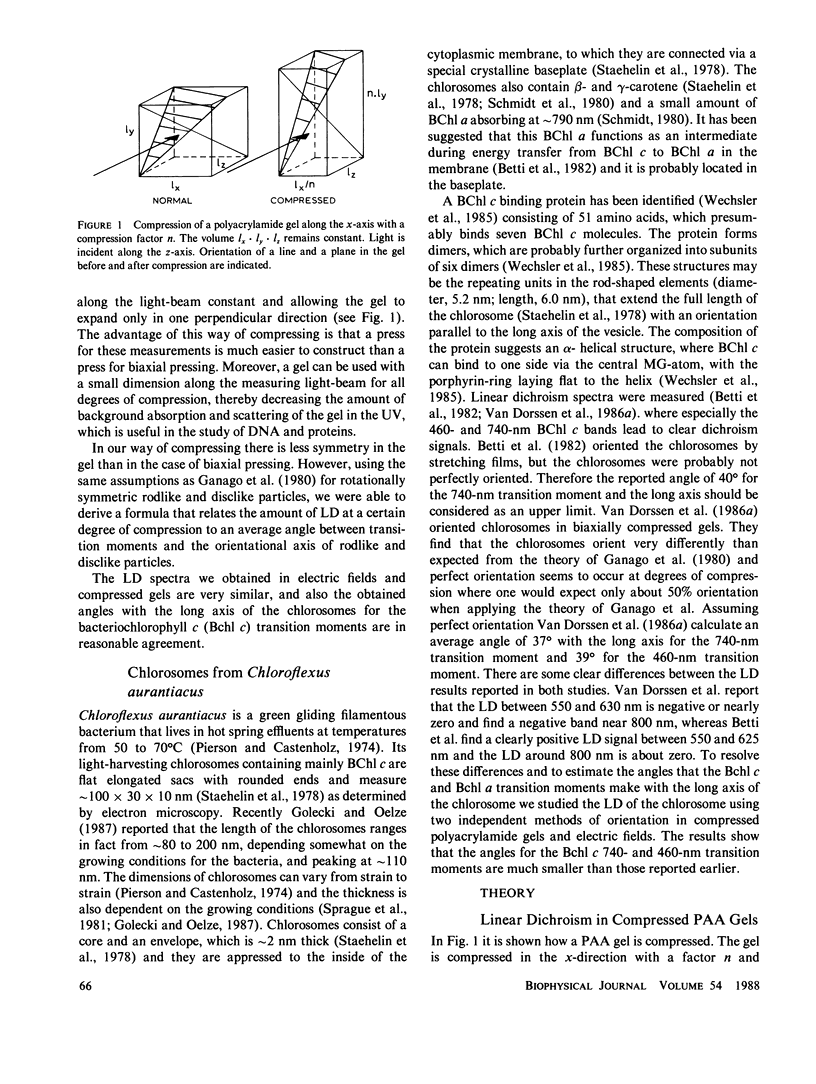
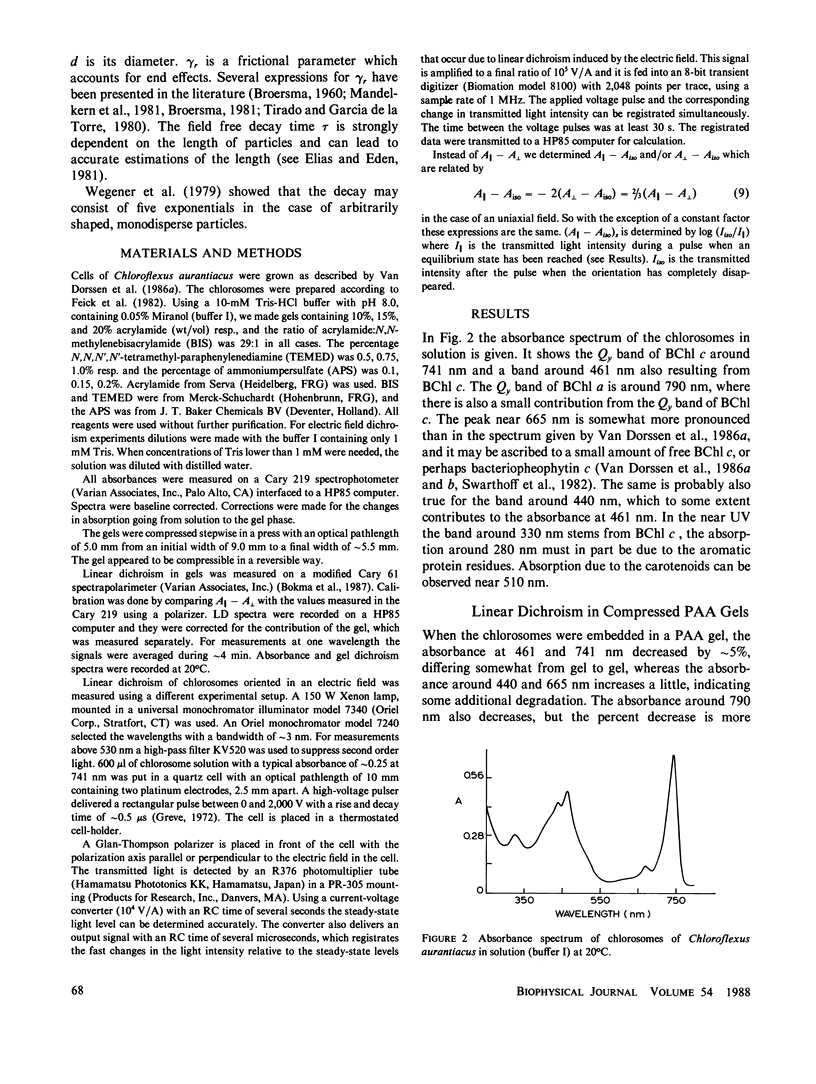
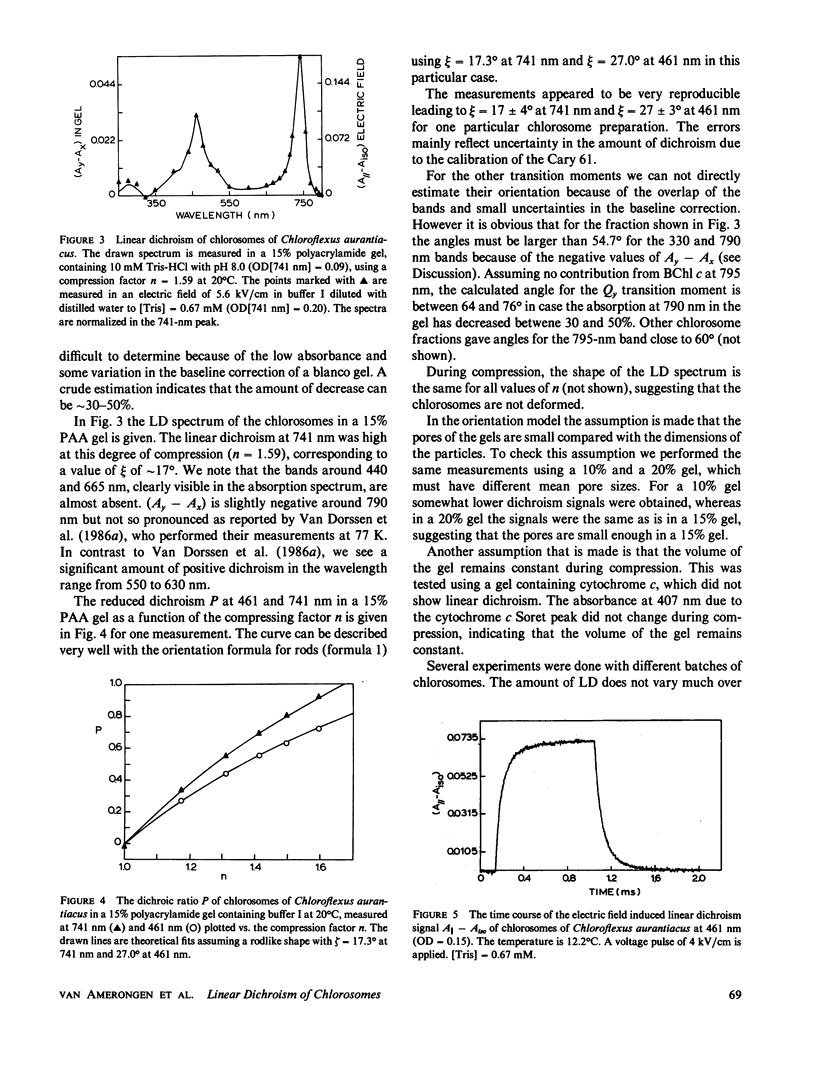
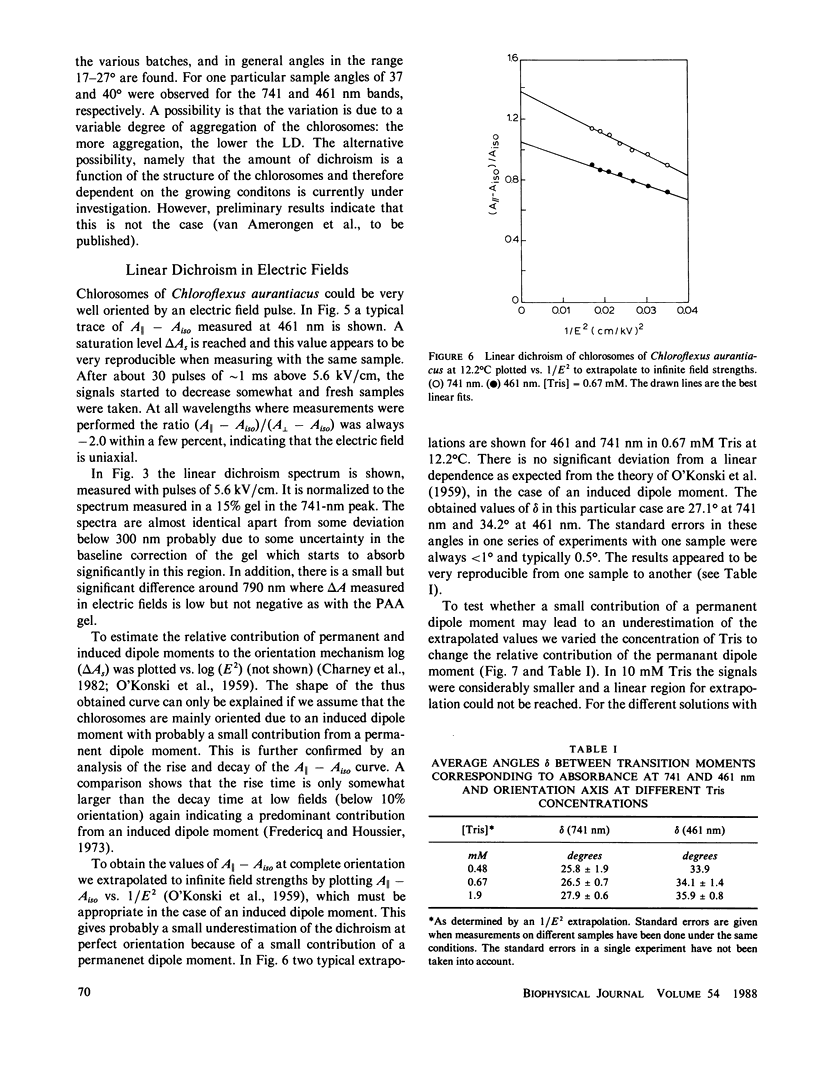
The linear dichroism of chlorosomes from Chloroflexus aurantiacus was measured between 250 and 800 nm. To orient the chlorosomes we used a new way of compressing polyacrylamide gels, where the dimension of the gel along the measuring light-beam is kept constant. The press required for such a way of compressing is relatively easy to construct. A theoretical description is given to interpret the measured linear dichroism in terms of the orientation of the transition moments. The results obtained with the polyacrylamide gels are compared with the linear dichroism measurements for chlorosomes oriented in electric fields. Both the spectral features as well as the absolute size of the linear dichroism signals are in reasonable agreement. We find that the transition moment corresponding to the 741 nm bacteriochlorophyll c (Bchl c) absorption band makes an angle of 20° with the long axis of the chlorosome. For the 461 nm Bchl c band an angle of 30° is found. Both angles are significantly lower than the values reported so far in literature and they imply that Bchl c is highly organized in the chlorosomes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdourakhmanov I. A., Ganago A. O., Erokhin Y. E., Solov'ev A. A., Chugunov V. A. Orientation and linear dichroism of the reaction centers from Rhodopseudomonas sphaeroides R-26. Biochim Biophys Acta. 1979 Apr 11;546(1):183–186. doi: 10.1016/0005-2728(79)90180-4. [DOI] [PubMed] [Google Scholar]
- Bokma J. T., Johnson W. C., Jr, Blok J. CD of the Li-salt of DNA in ethanol/water mixtures: evidence for the B- to C-form transition in solution. Biopolymers. 1987 Jun;26(6):893–909. doi: 10.1002/bip.360260609. [DOI] [PubMed] [Google Scholar]
- Charney E., Yamaoka K. Electric dichroism of deoxyribonucleic acid in aqueous solutions: electric field dependence. Biochemistry. 1982 Mar 2;21(5):834–842. doi: 10.1021/bi00534a004. [DOI] [PubMed] [Google Scholar]
- Feick R. G., Fitzpatrick M., Fuller R. C. Isolation and characterization of cytoplasmic membranes and chlorosomes from the green bacterium Chloroflexus aurantiacus. J Bacteriol. 1982 May;150(2):905–915. doi: 10.1128/jb.150.2.905-915.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganago A. O., Fok M. V., Abdurakhmanov I. A., Solov'ev A. A., Erokhin Iu E. Analiz lineinogo dikhroizma reaktsionnykh tsentrov, orientirovannykh v poliakrilamidnom gele. Mol Biol (Mosk) 1980 Mar-Apr;14(2):381–389. [PubMed] [Google Scholar]
- Mandelkern M., Elias J. G., Eden D., Crothers D. M. The dimensions of DNA in solution. J Mol Biol. 1981 Oct 15;152(1):153–161. doi: 10.1016/0022-2836(81)90099-1. [DOI] [PubMed] [Google Scholar]
- Pierson B. K., Castenholz R. W. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch Microbiol. 1974;100(1):5–24. doi: 10.1007/BF00446302. [DOI] [PubMed] [Google Scholar]
- Sprague S. G., Staehelin L. A., DiBartolomeis M. J., Fuller R. C. Isolation and development of chlorosomes in the green bacterium Chloroflexus aurantiacus. J Bacteriol. 1981 Sep;147(3):1021–1031. doi: 10.1128/jb.147.3.1021-1031.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


