Abstract
Host and bacterial factors that determine whether Salmonella serotypes remain restricted to the gastrointestinal tract or penetrate beyond the mucosa and cause systemic disease remain largely undefined. Here, factors influencing Salmonella host specificity in calves were assessed by characterizing the pathogenesis of different serotypes. Salmonella enterica serotype Dublin was highly virulent intravenously, whereas S. enterica serotype Choleraesuis was moderately virulent. Both serotypes were virulent in calves infected orally. In contrast, S. enterica serotypes Gallinarum and Abortusovis were avirulent by either route. Serotypes Dublin, Gallinarum, and Abortusovis colonized the intestinal tract 24 h after oral inoculation, yet only serotype Dublin was consistently recovered from systemic tissues. Serotypes Dublin and Gallinarum invaded bovine intestines in greater numbers and induced greater enteropathogenic responses than serotypes Choleraesuis and Abortusovis. However, only serotype Dublin was able to persist within the intestinal mucosa, and use of a novel cannulation model demonstrated that serotype Dublin was able to pass through the mesenteric lymph nodes in greater numbers than serotype Gallinarum. Together, these results suggest that initial interactions with the intestinal mucosa do not correlate with host specificity, although persistence within tissues and translocation via efferent lymphatics appear to be crucial for the induction of bovine salmonellosis.
Within the species Salmonella enterica there are >2,000 different serotypes, including bacteria of great veterinary and medical significance, that can result in symptoms ranging from mild enteritis to severe systemic disease. Virulent Salmonella serotypes can differ greatly in their degree of host specificity (7, 27, 43). At present the genetic and phenotypic characteristics that limit the pathogenicity of certain serotypes in particular hosts are not fully understood. In cattle, S. enterica serotype Dublin is considered to be the predominant host-restricted serotype (41, 42, 46). Other serotypes, for example, S. enterica serotype Typhimurium, may also cause disease in cattle, but in contrast to serotype Dublin, serotype Typhimurium is frequently associated with intestinal infections in a wide range of phylogenetically unrelated species (29). Serotype Dublin, however, may also cause disease in a limited range of other mammals, including sheep and humans (10, 15, 41, 42). Similarly, S. enterica serotype Choleraesuis, which is regarded as being host restricted to pigs, may also cause disease in a limited number of other species (1, 10). On the other hand host-specific S. enterica serotypes such as Typhi, Gallinarum, and Abortusovis are typically associated with systemic disease and often abortion in phylogenetically closely related species only, namely, humans (22), fowl (39), and sheep and goats (24), respectively.
Several factors that affect the virulence of a given serotype for a particular host have been proposed. These include intestinal invasion and destruction of the mucosal epithelium (35), avoidance of the induction of an intestinal inflammatory response (20, 32, 44), and survival within macrophages (47). In mice, serotype Typhimurium is more efficient at invading and destroying the intestinal epithelium than serotype Typhi (35), and serotype Typhimurium can persist in higher numbers than serotype Typhi in primary murine macrophages in vitro (47). These parameters correlate with the relative virulence of serotypes Typhimurium and Typhi in mice. However until the precise mechanisms of pathogenicity in different serotype-host combinations are fully understood, the importance of these two observations cannot be inferred. Furthermore, similar experiments quantifying the magnitude of intestinal invasion of different serotypes in cattle, pigs, and sheep (9, 44) and survival within porcine macrophages (53) clearly demonstrate that these parameters do not necessarily correlate with host specificity in other combinations of serotype and host.
The transmission of a given serotype to its specific host is also important in determining the incidence of salmonellosis (27). This has, however, received relatively little study. It is possible that the mechanism of transmission in diverse combinations of serotype and host may be different and may not directly correlate with virulence. For example, serotype Abortusovis infection of pregnant ewes typically has a low mortality rate, and animals may appear almost asymptomatic until the ewe aborts a heavily infected fetus, allowing the spread of the organism to other animals (24). Serotype Abortusovis is relatively poorly invasive and induces little damage in ovine intestines (44). Thus, this serotype could be described as having low virulence for adult sheep but high specificity attributable to its successful mode of transmission.
In order to elucidate the molecular basis of serotype-host specificity, one must consider as a whole the virulence, the multiple steps involved in pathogenesis, and the modes of transmission of different serotypes in a given host. In this study, the degrees of virulence of different Salmonella serotypes for cattle were compared. Salmonella-host interactions were characterized at various stages of pathogenesis, including initial colonization of tissues, intestinal invasion, induction of enteropathogenic responses, translocation to systemic sites, and survival within intestinal and systemic tissues. The results obtained in this study provide insight into the mechanisms involved in the ability of some serotypes, but not others, to be pathogenic in a given host.
MATERIALS AND METHODS
Bacterial strains.
The serotypes used in this study were as follows: Typhimurium, strains ST4/74 (26) and ST12/75 (4); Dublin, strains SD3246 (19) and SD2229 (4); Choleraesuis, strains SCSA50 and SCS14/74 (9, 53); Gallinarum, strains SG9 (54) and SGJ91 (12); and Abortusovis, strains SAO44 (13) and SAO15/5 (28). The virulence and magnitude of intestinal invasion of these strains have recently been characterized in sheep (44), pigs (53), and chickens (personal communication). The strains were stored as mid-log-phase cultures in Luria-Bertani (LB) broth containing 10% glycerol at −70°C and were streaked onto LB agar plates and incubated overnight at 37°C for use.
Calf infection studies.
All animal experiments were conducted according to the requirements of the Animal Scientific Procedures Act (1986). Friesian bull calves, aged approximately 28 days, were housed in a high containment unit and fed twice daily on a diet of reconstituted dried milk and unmedicated weaner nuts, with water freely available. All experimental animals were prescreened for the presence of Salmonella as described previously (49). None of the calves excreted Salmonella either at 1 week or immediately before infection. Salmonella cultures were prepared by inoculating Bacto Tryptose broth (25) with several bacterial colonies and incubating them statically at 37°C for 18 h. For oral inoculation, 8.9 log10 CFU were diluted in 20 ml of antacid solution [sterile double-distilled water containing 5% (wt/vol) Mg(SiO3)3, 5% (wt/vol) NaHCO3, and 5% (wt/vol) MgCO3]. The inoculum was administered to each calf with the aid of a syringe immediately before the morning feeding. For intravenous (i.v.) inoculation, cultures were diluted in 1 ml of isotonic saline to give a final concentration of 6.0 log10 CFU and injected directly into the jugular vein.
Following inoculation by either route, the animals were monitored for clinical signs of disease, and rectal temperatures were recorded twice daily. A cumulative daily scoring scheme was used to record the severity of diarrhea as described previously (48). Calves were killed with an overdose of barbiturate if predefined clinical end points (48) were met during the course of infection. The surviving animals were killed either 24 h or 7 days after inoculation. At postmortem examination, the numbers of bacteria present in the lung, spleen, liver, ileum, cecum, and colon and their associated lymph nodes, together with the intestinal contents, were determined by the viable-count method (48). Typically, bacteria were plated on modified brilliant green agar (CM 329; Oxoid, Basingstoke, Hampshire, United Kingdom) with the addition of 120 mg of sulfadiazine/liter, while serotype Abortusovis SAO44 was plated on Rambach agar (Merck Ltd., Lutterworth, Leicestershire, United Kingdom). The mucosa and contents of the rumen, reticulum, omasum, and abomasum were sampled, in addition to the tissues listed above, in calves killed after 24 h. The limit of accurate quantification of the viable-count method is 2.0 log10 CFU/g of tissue. Any samples containing fewer bacteria were enriched in Rappaport and selenite brilliant green broth (49). Samples that were positive on enrichment were assumed to contain between 1 and 100 CFU/g and were given a value of 2.0 log10 CFU/g, while those that were negative were given a value of 0.
Bovine ligated-ileal-loop model for quantification of intestinal invasion, enteropathogenesis, and bacterial persistence.
Modifications to the bovine ligated-ileal-loop model used by Watson et al. (51), Wallis et al. (48), and Bispham et al. (8) were employed to quantify intestinal invasion, enteropathogenesis (secretory and inflammatory responses), and bacterial persistence, respectively. Salmonella cultures were incubated overnight at 25°C with agitation, diluted approximately 1 in 3 in fresh LB broth, and incubated for a further 2 h at 37°C with agitation. The bacterial concentration was adjusted, by the addition of LB broth, to 6.7 log10 CFU/ml for the invasion and persistence assays and 8.3 log10 CFU/ml for the enteropathogenesis experiments. The calves were anesthetized, and intestinal loops either 9 (invasion and persistence) or 6 cm (enteropathogenesis) long, spaced 1 cm apart, were constructed in the distal or midileum, respectively, using surgical silk. In calves, the distal ileum contains a long strip of continuous Peyer's patch (34), and so a direct evaluation of the relative invasiveness of different serotypes for Peyer's patch mucosa and absorptive epithelium could be made. A total of 5 ml of Salmonella suspension was injected into each test loop, and the same volume of sterile LB broth was used as a negative control. For quantifying bacterial invasion and persistence, the loops were exteriorized after 1 h, and 5 ml of solution GC/Tcm10 (3) containing 300 μg of gentamicin/ml was injected into each loop prior to reinternalization of the loop and repair of the wound. The invasion loops were left in situ for another hour, while those used for determination of bacterial persistence were left in situ for another 10 h. Different calves were used in experiments assessing either bacterial invasion or persistence. After the appropriate incubation period, the calves were killed with an overdose of barbiturate. The numbers of bacteria present in circular biopsy specimens of 6-mm radius were determined by the viable-count method as described previously (51).
For quantifying enteropathogenesis, 50 ml of blood was withdrawn immediately after loop inoculation and mixed with 9 ml of acid-citrate (trisodium citrate, 85 mM; citric acid, 46 mM; pH 4.9). Polymorphonuclear leukocytes (PMN) were isolated using the method of Carlson and Kaneko (11) and labeled with 111In-oxinate (Mallinckrodt, Petten, The Netherlands) as described previously (48). At 12 h after incubation, the calves were killed with an overdose of barbiturate and the loops were removed. The secretory responses were defined as the ratio of fluid within the loop lumen to the length of the loop. The PMN influx was defined as the ratio of γ emission from radiolabeled PMN within the test loop mucosa and contents to that from the negative control loops.
Cannulation of efferent lymph vessels from mesenteric lymph nodes draining infected ileal loops.
Inocula were prepared at a concentration of ∼8.9 log10 CFU/ml, and calves were prepared for surgery as described above. A single ligated ileal loop of 80 cm flanked by two loosely tied spacers was constructed in the distal ileum. An efferent lymphatic vessel from a mesenteric lymph node draining the ligated section of the intestine was cannulated. The area of mesentery containing the cannulated vessel was sutured onto a piece of corrugated plastic; the ligated ileal loop, together with the attached cannula, was replaced within the abdominal cavity; and the wound was repaired. Preinfection lymph was collected continuously for 2 h at room temperature in tubes containing heparin at 50 U/ml. The distal ileum was exteriorized, and the ligated loop was inoculated with 50 ml of the test strain via the loosely tied spacers prior to loop replacement and wound repair. Care was taken not to inject straight into the loop in order to reduce the accidental administration of bacteria directly into a blood vessel. Lymph was collected continuously for another 10 h as described above, and the number of bacteria present in lymph collected over 2-h periods was determined by the viable-count method.
Statistical analyses.
All viable-count data were normalized by logarithmic transformation. Unless otherwise stated, data were statistically analyzed using a two-way analysis of variance and the general linear model (software supplied by Minitab Inc., State College, Pa.) for the effect of the serotype and, when appropriate, the strain on the particular assay used. Subsequently, pairwise comparisons between strains and serotypes were performed. In the event that any differences were found, the Student t test was applied. The variance used in the t test was obtained from the adjusted mean square calculated in the analysis of variance. Standard errors of the mean in the histograms are based on an estimate of the variance calculated from the observations for each group and were not calculated using the pooled variance from the analysis of variance. The effects of serotype on rectal temperature were tested by means of an F test with the data taken as repeated measurements (Procedure Mixed [Statistical Analysis Systems; SAS Institute, Cary, N.C.]). Analyses of data from the cannulation and persistence assays were performed by means of paired t tests (Minitab Inc.).
RESULTS
Virulence of serotypes Dublin, Gallinarum, and Abortusovis, but not serotype Choleraesuis, in orally inoculated calves correlates with their predicted specificities for cattle.
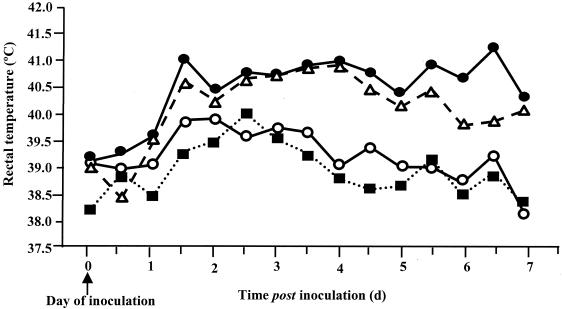
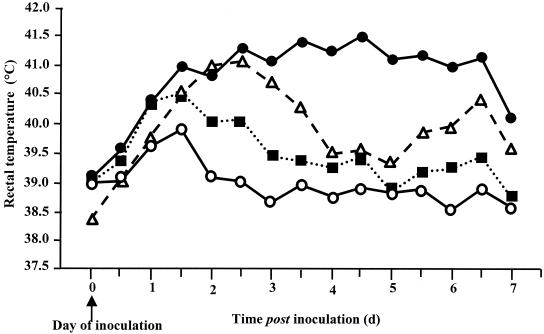
The virulence of serotypes Dublin, Choleraesuis, Gallinarum, and Abortusovis in cattle was assessed by daily monitoring of rectal temperatures and the production and severity of diarrhea for up to 7 days after oral inoculation. Bacterial recovery from selected systemic and enteric sites was determined following postmortem examination. All four Salmonella serotypes induced an increase in rectal temperature 1 to 2 days after inoculation. This response was severe and prolonged in six calves per group inoculated with either serotype Dublin or Choleraesuis but mild and transient in three calves per group inoculated with either serotype Gallinarum or Abortusovis (Fig. 1). From 2 days after inoculation, the rectal temperatures induced by serotypes Dublin and Choleraesuis were greater than those induced by serotype Gallinarum or Abortusovis (P, <0.07 to <0.001). Only calves inoculated with serotype Dublin or Choleraesuis became dull and anorexic and developed diarrhea. Three out of six calves infected with each of these serotypes reached predefined clinical endpoints before 7 days after inoculation (Table 1) and were therefore humanely killed as indicated in Materials and Methods. At postmortem examination, only animals infected with either serotype Dublin or Choleraesuis had intestinal changes typical of acute salmonellosis, including necrotic, reddened ileal and colonic mucosa and enlarged, necrotic mesenteric lymph nodes. In general, serotypes Dublin and Choleraesuis were recovered from systemic and intestinal tissues in comparable numbers. Serotypes Gallinarum and Abortusovis were recovered from the intestinal mucosa and lymph nodes by enrichment culture only and were not recovered at all from intestinal contents or systemic tissues (Table 2). These data demonstrate that serotypes Dublin and Choleraesuis are more virulent than serotypes Gallinarum and Abortusovis following oral inoculation of calves. With the exception of serotype Choleraesuis, these data correlate with the degree of specificity of these serotypes in cattle based on epidemiological data (41, 42). Thus, it can be concluded that infection of cattle with different serotypes is a useful model to explore possible factors contributing to the low virulence of particular serotypes in a given host.
FIG. 1.
Least-squares mean daily pyrexic responses following oral inoculation of calves with serotype Dublin SD3246 (•), Choleraesuis SCSA50 (▵), Gallinarum SG9 (○), or Abortusovis SAO44 (▪). Each datum point is derived from the mean of six SD3246- or SCSA50-infected calves per group and three SG9- or SAO44-infected calves per group. The SEM for SD3246 and SCSA50 is 0.317, while the SEM for SG9 and SAO44 is 0.351.
TABLE 1.
Clinical findings for calves inoculated by the oral route with S. enterica serotype Dublin, Choleraesuis, Gallinarum, or Abortusovis
| Strain | No. surviving/ no. inoculated | Mean time of euthanasia (days) ± SEM | Mean diarrhea score ± SEM |
|---|---|---|---|
| SD3246 | 3/6 | 5.8 ± 0.6 | 12.7 ± 4.2 |
| SCSA50 | 3/6 | 5.3 ± 0.8 | 11.8 ± 2.3 |
| SG9 | 3/3 | 7.0 ± 0.0 | 0.7 ± 0.7 |
| SAO44 | 3/3 | 7.0 ± 0.0 | 0.3 ± 0.3 |
TABLE 2.
Recovery of S. enterica serotypes Dublin, Choleraesuis, Gallinarum, and Abortusovis from selected systemic and enteric sites up to 7 days after oral inoculation of calvesa
| Tissue or material | Bacterial recovery (log10 CFU/ml ± SEM)
|
|||
|---|---|---|---|---|
| SD3246 | SCSA50 | SG9 | SAO44 | |
| Blood | 0 | 0 | 0 | 0 |
| Liver | 3.02 ± 0.21 | 2.31 ± 0.55 | 0 | 0 |
| HLN | 2.62 ± 0.23 | 2.89 ± 1.11 | 0 | 0 |
| Lung | 3.48 ± 0.24 | 3.15 ± 0.34 | 0 | 0 |
| MDLN | 2.42 ± 0.19 | 2.01 ± 0.48 | 0 | 0 |
| Spleen | 2.94 ± 0.22 | E (3/6) | 0 | 0 |
| Intestinal contents | ||||
| Ileum | 2.18 ± 1.13 | 4.94 ± 0.85 | 0 | 0 |
| Cecum | 4.35 ± 0.99 | 5.23 ± 0.64 | 0 | 0 |
| Colon | 3.63 ± 1.38 | 5.37 ± 0.69 | 0 | 0 |
| Intestinal mucosa | ||||
| Ileum | 4.47 ± 0.69 | 5.80 ± 0.72 | E (1/3) | E (1/3) |
| Cecum | 5.05 ± 0.32 | 5.54 ± 0.62 | E (2/3) | E (2/3) |
| Colon | 4.54 ± 0.38 | 4.79 ± 1.12 | E (2/3) | 0 |
| Mesenteric lymph nodes | ||||
| Ileum | 4.30 ± 0.41 | 4.75 ± 1.07 | E (1/3) | E (1/3) |
| Cecum | 4.67 ± 0.21 | 5.40 ± 0.63 | E (3/3) | E (2/3) |
| Colon | 4.12 ± 0.31 | 4.85 ± 1.05 | 2.43 ± 0.43 | E (1/3) |
Triplicate samples were taken from each tissue or material, and means were calculated to give a value per animal. Each figure represents the mean of six calves each for serotypes Dublin (SD3246) and Choleraesuis (SCSA50) and three calves each for serotypes Gallinarum (SG9) and Abortusovis (SAO44) with the SEM. The limit of accurate quantification was 2.0 log10 CFU/g, and samples that contained salmonellae in numbers too low to quantify are indicated by E (positive on enrichment culture) with the number of positive tissues/number of calves used in parentheses. HLN, hepatic lymph node; MDLN, mediastinal lymph node.
Serotype Dublin colonizes both intestinal and systemic sites in greater numbers than either serotype Gallinarum or Abortusovis during the initial stages of infection.
To determine whether Salmonella serotypes, which differ in virulence for cattle, initially colonized the intestinal mucosa in similar numbers, two groups of five calves and one group of three calves were inoculated orally with serotype Dublin, Gallinarum, or Abortusovis, respectively. Bacteria were subsequently recovered from systemic and intestinal tissues 24 h after oral inoculation. Bacterial recovery from the stomach (omasum, abomasum, rumen, and reticulum) contents or mucosa was low, with serotypes Dublin and Gallinarum being occasionally isolated after enrichment culture only. However, serotype Dublin was the only serotype reproducibly recovered by enrichment culture from the majority of systemic tissues. Serotype Dublin was recovered in greater numbers than serotype Abortusovis from the ileum, cecum, and colon (P, <0.1 to <0.01) and in greater numbers than serotype Gallinarum from these sites (P, <0.1 to <0.01), with the exception of the lymph nodes associated with the ileum and cecum. In these tissues, despite the greater recovery of serotype Dublin, bacterial numbers in serotype Gallinarum-infected calves were statistically similar (P > 0.1) (Table 3). Serotype Gallinarum was recovered in numbers similar to those of serotype Abortusovis from all sites tested (P > 0.1). These data show that all three serotypes are able to colonize the intestinal mucosa and nodes. Therefore, the low rate of recovery of serotypes Gallinarum and Abortusovis from intestinal and systemic sites 7 days after oral inoculation cannot be accounted for by the killing of bacteria during passage through the stomachs and intestines. However, the reduced colonization of serotypes Gallinarum and Abortusovis correlated with the avirulence of these serotypes following oral inoculation. Possible reasons for this observation were investigated further.
TABLE 3.
Recovery of S. enterica serotypes Dublin, Gallinarum, and Abortusovis from selected enteric and systemic sites 24 h after oral inoculation of calvesa
| Tissue or material | Bacterial recovery (log10 CFU/ml ± SEM)
|
||
|---|---|---|---|
| SD3246 | SG9 | SAO44 | |
| Liver | E (5/5) | E (2/5) | E (0/3) |
| HLN | E (5/5) | E (0/5) | E (0/3) |
| Lung | E (4/5) | E (2/5) | E (0/3) |
| MDLN | E (2/5) | E (0/5) | E (0/3) |
| Spleen | E (5/5) | E (2/5) | E (0/3) |
| Intestinal contents | |||
| Ileum | 4.53 ± 0.61 | 2.88 ± 0.25 | E (2/3) |
| Cecum | 4.79 ± 0.42 | 3.01 ± 0.28 | E (2/3) |
| Colon | 5.14 ± 0.45 | 3.14 ± 0.27 | E (2/3) |
| Intestinal mucosa | |||
| Ileum | 5.00 ± 0.43 | 3.87 ± 0.28 | 3.02 ± 0.36 |
| Cecum | 5.21 ± 0.21 | 3.89 ± 0.15 | 3.51 ± 0.76 |
| Colon | 3.89 ± 0.26 | 2.51 ± 0.21 | 2.57 ± 1.29 |
| Mesenteric lymph nodes | |||
| Ileum | 4.67 ± 0.27 | 4.2 ± 0.03 | 3.28 ± 0.42 |
| Cecum | 4.78 ± 0.2 | 4.13 ± 0.1 | 3.25 ± 0.64 |
| Colon | 4.5 ± 0.3 | 2.87 ± 0.39 | 3.26 ± 0.66 |
Triplicate samples were taken from each tissue or material, and means were calculated to give a value per animal. Each figure represents the mean of five calves each for serotypes Dublin (SD3246) and Gallinarum (SG9) and three calves for serotype Abortusovis (SAO44) with the SEM. The limit of accurate quantification was 2.0 log10 CFU/g, and samples that contained salmonellae in numbers too low to quantify are indicated by E (positive on enrichment culture) with the number of positive tissues/number of calves used parentheses. HLN, hepatic lymph node; MDLN, mediastinal lymph node.
Intestinal invasion and induction of enteropathogenic responses does not correlate with virulence of Salmonella serotypes following oral inoculation.
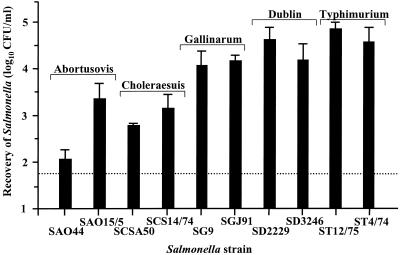
Salmonella colonization of the intestines, as measured 24 h after oral inoculation, is affected by both initial intestinal invasion and subsequent bacterial persistence and growth. The contribution of intestinal invasion to both colonization and virulence was investigated using the bovine ligated-ileal-loop model. Between 6 and 12 individual loops per Salmonella strain were infected for 1 h, gentamicin was injected, and the loops were left for a further 1-h incubation prior to determination of the number of bacteria present within the mucosa by the viable-count method. The ligated-ileal-loop model allows several strains to be tested within one animal, and thus, it was possible to include two strains for each serotype. Serotype Typhimurium was also included in these experiments to allow a comparison to previous work performed in cattle (9, 51), pigs (9), sheep (44), and chickens (personal communication). For simplicity of interpretation, significance levels were determined from the mean values for two strains within a given serotype. The relative levels of invasiveness of the different serotypes for Peyer's patch mucosa and absorptive epithelia were also compared. As there were no significant strain differences in bacterial recovery from mucosa either with or without Peyer's patches or from mucosa in the distal compared to the midileum, results are presented based on data derived from the absorptive epithelia in the distal ileum only (Fig. 2). On average, serotype Typhimurium was more invasive than serotype Dublin (P < 0.01). These two serotypes were more invasive than serotype Gallinarum (P, <0.05 to <0.01), while serotype Choleraesuis was less invasive than serotype Gallinarum (P < 0.01) but more invasive than serotype Abortusovis (P < 0.05). The structural integrity of the intestinal mucosa was maintained during the infection, as assessed by microscopy (unpublished data), indicating that bacterial recovery would be unlikely to be affected by shedding of infected host cells or uptake of gentamicin, both of which may result from mucosal damage. These data show that serotypes Typhimurium, Dublin, and Gallinarum are more invasive in the bovine ileum than serotype Choleraesuis or Abortusovis, despite serotype Choleraesuis being more virulent than serotype Gallinarum following oral inoculation. In addition, the lower number of serotype Gallinarum cells than serotype Dublin cells able to colonize the bovine gastrointestinal tract after 24 h does not correlate with the intestinal invasion of this serotype 2 h after infection of ligated ileal loops.
FIG. 2.
Magnitudes of intestinal invasion of different Salmonella serotypes 2 h after infection of bovine ligated ileal loops. The results shown were derived from ligated loops constructed in the distal ileum containing absorptive epithelia. Triplicate samples were taken from two or three loops in each animal, and means were calculated to give a value per calf. Each bar represents the mean of two, three, or four calves plus the SEM. The dotted line represents the limit of accurate quantification.
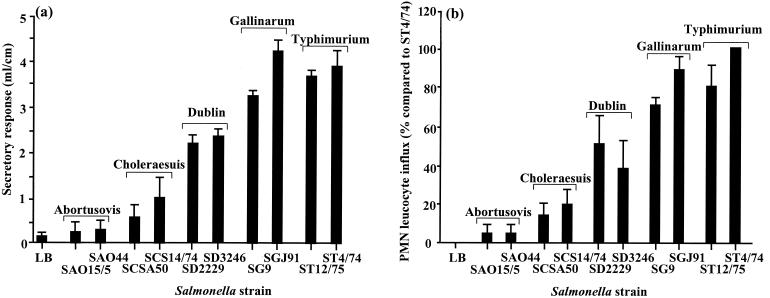
The relationship between the enteropathogenicity and virulence of the different serotypes was investigated, again using the bovine ligated-ileal-loop model. Between 8 and 10 individual loops per strain were infected with Salmonella, and both the secretory response and PMN influx into the lumen and mucosa were quantified after 12 h. In order to obtain an average PMN influx for all calves, the results per animal were expressed as the mean percentage minus 1 (the value for the LB control loops) compared to the mean value for serotype Typhimurium ST4/74 (the strain that induced the greatest response) (Fig. 3). For simplicity of interpretation, significance levels were determined from the mean values for two strains within a given serotype. Serotypes Typhimurium and Gallinarum induced similar secretory responses (P > 0.1), while the PMN influx induced by serotype Typhimurium was greater than that induced by serotype Gallinarum (P < 0.05). These two serotypes induced greater secretory and inflammatory responses than serotype Dublin (P < 0.001), which in turn induced greater responses than serotype Choleraesuis (P < 0.001). Serotype Abortusovis induced little or no secretory response or influx of inflammatory cells. The induction of enteropathogenic responses by the different serotypes clearly does not correlate with their relative virulence in cattle; however, there was a direct correlation between the magnitudes of the secretory and inflammatory responses. Furthermore, there was no direct correlation between the magnitude of enteropathogenic responses and intestinal invasion, as despite being similarly invasive, serotype Gallinarum was significantly more enteropathogenic than serotype Dublin, indicating that serotype-specific factors independent of intestinal invasion influence enteropathogenicity. Therefore, further studies were carried out using these two serotypes.
FIG. 3.
Secretory and inflammatory responses induced by different Salmonella serotypes 12 h after infection of bovine ligated ileal loops. Each bar represents the mean response, per animal, derived from 2 or 3 loops per strain in three calves (total, 8 to 10 loops) plus the SEM. The secretory response (a) represents the mean volume/length ratio, and the PMN influx (b) is presented as a percentage compared to serotype Typhimurium ST4/74.
Bacterial translocation of serotypes Dublin and Gallinarum from mesenteric lymph nodes correlates with their virulence following oral inoculation.
Both serotypes Dublin and Gallinarum initially colonize and invade bovine ileum and lymph nodes in relatively high numbers. However, only serotype Dublin results in severe systemic disease in calves, which is reflected by the greater frequency with which serotype Dublin colonizes systemic sites, compared with serotype Gallinarum, following oral inoculation.
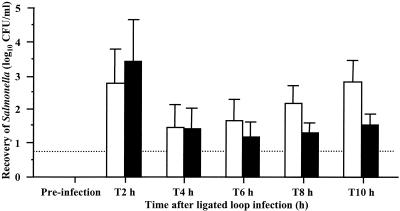
The translocation of serotypes Dublin and Gallinarum from ileal lymph nodes was quantified in four calves per serotype, using a novel cannulation model. An efferent lymph vessel from a node directly draining an area of infected intestinal mucosa was cannulated, and the number of bacteria within the lymph was determined by the viable-count method over a 10-h period (Fig. 4). The initial recovery rates of serotypes Dublin and Gallinarum were similar (P > 0.1), with a peak of approximately 3.0 log10 CFU/ml at 2 h falling to approximately 1.5 log10 CFU/ml at 4 and 6 h. However, from 8 and 10 h post-loop infection, serotype Dublin was recovered in significantly greater numbers than serotype Gallinarum (P < 0.05). These results demonstrate the potential of serotype Dublin to disseminate in higher numbers than serotype Gallinarum and correlate with systemic colonization and virulence following the oral inoculation of calves.
FIG. 4.
Recovery of serotypes Dublin SD3246 (open bars) and Gallinarum SG9 (solid bars) from efferent lymph up to 10 h after infection of bovine ligated ileal loops. Each bar represents the mean of individual lymph samples from four calves per serotype plus the SEM. The dotted line represents the limit of accurate quantification.
Persistence of serotypes Dublin and Gallinarum within bovine ileal mucosa correlates with their virulence.
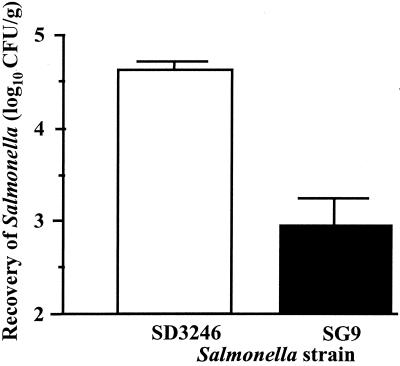
The relative persistence of serotypes Dublin and Gallinarum in bovine tissue was assessed in ligated ileal loops. The loops were infected with either serotype for 1 h, as for the invasion assay, and then incubated with gentamicin for another 10 h, at which point the number of bacteria within the mucosa was determined by the viable-count method. Recovery of serotype Dublin was significantly higher than recovery of serotype Gallinarum (P < 0.01). The results shown (Fig. 5) are from a representative calf, with similar results obtained from two other animals (unpublished data). Recovery of serotype Dublin had increased by approximately 0.5 log10 CFU/ml compared to the recovery in loops incubated for only 1 h with gentamicin, whereas the recovery of serotype Gallinarum fell by approximately 1.0 log10 CFU/ml (Fig. 2). These results demonstrate that serotype Dublin is able to persist within bovine intestinal mucosa in greater numbers than serotype Gallinarum.
FIG. 5.
Recovery of serotypes Dublin SD3246 and Gallinarum SG9 11 h after infection of bovine ligated ileal loops. Each bar represents the mean of three loops per serotype in a single animal plus the SEM based on the mean counts per serotype.
Recovery of serotypes Dublin, Gallinarum, and Abortusovis, but not Choleraesuis, from host tissues after i.v. inoculation correlates with virulence after oral inoculation.
The persistence of bacteria within systemic tissues of three calves per Salmonella serotype was assessed 7 days after i.v. inoculation. Infection by this route allows direct bacterial access to systemic tissues, and any influence of the initial interaction with the intestines will have been bypassed. As none of the calves reached any of the predefined clinical endpoints, all of the animals were humanely killed 7 days after inoculation. All four Salmonella serotypes induced an increase in rectal temperature 1 to 2 days after inoculation. From 2 days onward, this increase was greater in calves infected with serotype Dublin than in calves infected with serotype Gallinarum or Abortusovis (P, <0.05 to <0.001). Infection with serotype Choleraesuis resulted in a response similar to that to serotype Dublin for up to 3 days after inoculation, followed by a drop and then a further rise in temperature toward the end of the experiment (Fig. 6). Calves inoculated with serotype Dublin or Choleraesuis became dull, lethargic, and anorexic after approximately 36 h. These symptoms, accompanied by diarrhea, persisted with different magnitudes of severity in calves inoculated with serotype Dublin but not in those inoculated with serotype Choleraesuis. Serotypes Choleraesuis, Gallinarum, and Abortusovis did not induce any diarrhea. At postmortem examination, serotype Dublin was recovered in greater numbers than serotype Choleraesuis from the intestinal contents, intestinal mucosa, and systemic tissues (P, <0.1 to <0.05) and in similar numbers from the intestinal lymph nodes and the lymph nodes associated with the systemic tissues (P > 0.1). Serotype Gallinarum was detected, by enrichment culture only, from a limited number of systemic and enteric tissues, while serotype Abortusovis was not recovered from any site sampled (Table 4). Bacterial recovery of serotypes Dublin, Gallinarum, and Abortusovis following i.v. inoculation correlates with virulence and bacterial recovery after oral inoculation. Despite being able to invade and translocate from the intestinal mucosa, serotype Gallinarum is unable to persist within systemic tissues, and this correlates with its low virulence in calves. In contrast, despite being less invasive, less enteropathogenic, and only moderately virulent when administered by the i.v. route, serotype Choleraesuis is as virulent as serotype Dublin following oral inoculation. These results suggest that the pathogenesis of serotype Choleraesuis is quite distinct from that of serotype Dublin.
FIG. 6.
Least-squares mean daily pyrexic responses following i.v. inoculation of calves with serotype Dublin SD3246 (•), Choleraesuis SCSA50 (▵), Gallinarum SG9 (○), or Abortusovis SAO44 (▪). Each datum point is derived from the mean of three calves per serotype. The SEM for all serotypes is 0.302.
TABLE 4.
Recovery of S. enterica serotypes Dublin, Choleraesuis, Gallinarum, and Abortusovis from selected systemic and enteric sites 7 days after i.v. inoculation of calvesa
| Tissue or material | Bacterial recovery (log10 CFU/ml ± SEM)
|
|||
|---|---|---|---|---|
| SD3246 | SCSA50 | SG9 | SAO44 | |
| Blood | E (1/3) | 0 | 0 | 0 |
| Liver | 3.90 ± 0.48 | E (1/3) | 0 | 0 |
| HLN | 3.37 ± 0.09 | 3.63 ± 0.07 | E (1/3) | 0 |
| Lung | 3.93 ± 0.51 | 2.57 ± 0.23 | 0 | 0 |
| MDLN | 3.05 ± 0.47 | 3.76 ± 0.21 | E (1/3) | 0 |
| Spleen | 3.84 ± 0.46 | E (2/3) | 0 | 0 |
| Intestinal contents | ||||
| Ileum | 4.24 ± 2.13 | 2.24 ± 0.24 | E (1/3) | 0 |
| Cecum | 5.21 ± 1.62 | 2.42 ± 0.42 | 0 | 0 |
| Colon | 5.16 ± 1.58 | 2.48 ± 0.48 | 0 | 0 |
| Intestinal mucosa | ||||
| Ileum | 4.45 ± 1.21 | E (2/3) | 0 | 0 |
| Cecum | 4.37 ± 1.19 | 2.50 ± 0.27 | 0 | 0 |
| Colon | 4.42 ± 1.24 | E (2/3) | 0 | 0 |
| Mesenteric lymph nodes | ||||
| Ileum | 4.13 ± 0.43 | 3.49 ± 0.25 | E (1/3) | 0 |
| Cecum | 3.85 ± 0.53 | 3.63 ± 0.24 | 0 | 0 |
| Colon | 3.67 ± 0.84 | 3.02 ± 0.51 | 0 | 0 |
Triplicate samples were taken from each tissue or material, and means were calculated to give a value per animal. Each figure represents the mean of three calves with the SEM. The limit of accurate quantification was 2.0 log10 CFU/g, and samples that contained salmonellae in numbers too low to quantify are indicated by E (positive on enrichment culture) with the number of positive tissues/number of calves used in parentheses. HLN, hepatic lymph node; MDLN, mediastinal lymph node.
DISCUSSION
The early host-pathogen interactions that influence the outcome of infection with different Salmonella serotypes remain largely undefined. In this study, factors that determine whether Salmonella serotypes remain restricted to the gastrointestinal tract or penetrate beyond the mucosa and cause systemic disease in calves were assessed. Bacterial persistence, in both enteric and systemic sites, appeared to be crucial in determining the virulence of serotype Gallinarum in calves, and this finding correlated with bacterial dissemination from the intestines to systemic tissues.
Serotypes Dublin and Choleraesuis were highly virulent in orally inoculated calves, while serotypes Gallinarum and Abortusovis had low virulence. These results confirm the expected specificities of serotypes Dublin, Gallinarum, and Abortusovis, but not serotype Choleraesuis (41, 42), in this host. In addition, they extend observations made in previous studies showing that the same strains of serotype Choleraesuis, Gallinarum, or Abortusovis used in this study are virulent in pigs (53), chickens (personal communication), and lambs (44), respectively. Serotype Choleraesuis has previously been reported to be highly virulent in orally inoculated calves (40), so although our result is surprising, it is not without precedent. Infection of calves with either serotype Gallinarum or Abortusovis provides an excellent model to explore possible factors that may contribute to their low virulence in different animal hosts.
It is interesting to consider why serotype Choleraesuis is not associated with natural infection of calves despite being virulent and inducing severe diarrhea following experimental oral inoculation. One possible explanation is that serotype Choleraesuis is unable to circulate within or gain access to the bovine population in sufficient numbers to cause disease. Both fecal shedding from carrier animals and uptake into the nasal cavity as a result of the behavior and method of feeding of pigs are important in maintaining serotype Choleraesuis in pig herds (6, 17). Nasal inoculation results in more severe disease than oral inoculation, and so the infective dose for pigs by the nasal route may be lower. It could therefore be speculated that serotype Choleraesuis might be maintained in numbers high enough to infect pigs but too low to infect calves. An alternative explanation is that induction of infectious diarrhea in calves is insufficient to maintain serotype Choleraesuis on a long-term basis within the bovine population. Serotype Dublin is also able to establish a carrier state in adult animals, and this may act as a continual source of infection for calves. If serotype Choleraesuis is unable to establish its own carriage in cattle, this may limit its maintenance within the bovine population.
It has been proposed that serotype Gallinarum is avirulent in mice due to its inability to invade the murine intestinal tract (35). Here, we have shown that serotypes Dublin, Gallinarum, and Abortusovis are able to colonize and invade bovine intestines. This is evident from the recovery of bacteria from the mucosa and draining lymph nodes 24 h after inoculation and is suggested by an increase in rectal temperature 36 h after oral inoculation. Previously, a ligated-ileal-loop assay has been used to determine the ability of serotype Typhimurium to induce inflammatory and secretory responses in calves (49, 51, 52). The results obtained in this study confirm the reported highly invasive and highly enteropathogenic phenotypes of this serotype in calves. Intestinal invasion and the induction of inflammatory and secretory responses are key phases in the pathogenesis of serotypes Dublin and Typhimurium, as demonstrated by characterization of the phenotypes of isogenic mutants in bovine ligated ileal loops and orally inoculated calves. Strains that were noninvasive or nonenteropathogenic in ileal loops were attenuated following oral inoculation of calves (49, 51). Conversely, mutations that did not reduce enteropathogenic responses in ligated loops did not affect the severity of enteritis in orally infected calves (48). To our surprise, serotype Gallinarum proved to be as enteropathogenic as serotype Typhimurium and almost as invasive, yet more enteropathogenic, than serotype Dublin. Therefore, it is evident that serotype Gallinarum has the virulence determinants required for penetrating mammalian intestinal mucosa. However, for serotype Gallinarum, intestinal invasion and enteropathogenesis in ligated ileal loops do not correlate with virulence or serotype-host specificity. Clearly, the virulence factors necessary for invasion and enteropathogenesis in loops are not sufficient to induce disease in calves following oral infection. It is probable that in orally inoculated calves serotype Gallinarum is unable to grow or persist within the intestines to reach sufficient numbers to induce overt enteric disease. Interestingly, there have been some reports of serotype Gallinarum associated with outbreaks of enteritis in mammalian species, including humans (10, 18, 41). Most of these reports were published prior to the successful elimination of serotype Gallinarum from United Kingdom poultry flocks in the 1960s, and thus, it is possible that sufficiently high numbers of bacteria to cause disease entered the food chain. In complete contrast to serotype Gallinarum, serotype Choleraesuis is relatively poorly invasive and induces either low or no enteropathogenic responses in both calves and pigs, yet it is virulent following experimental oral and i.v. infection of both of these hosts (references 40 and 53, this study, and unpublished data). This is apparently analogous to the phenotypes of serotype Abortusovis in sheep (44), serotype Gallinarum in chickens (20), and serotype Typhi in humans (14, 33), where systemic pathogenesis in the natural target host species appears to correlate with the absence of an initial intestinal inflammatory response following invasion.
It would seem that factors other than those influencing intestinal invasion and elicitation of intestinal inflammatory and secretory responses contribute to Salmonella serotype-host specificity in calves. We have therefore developed a novel experimental model to study dissemination of Salmonella from intestinal to systemic sites. Both serotypes Dublin and Gallinarum were invasive and enteropathogenic yet exhibited contrasting virulence following oral or i.v. inoculation. Cannulation of efferent lymph vessels from mesenteric lymph nodes draining an infected ligated ileal loop demonstrated that serotypes Dublin and Gallinarum were initially able to translocate from the intestinal lumen to systemic sites in similar numbers. However, while the total number of disseminating bacteria decreased from 6 h after loop infection, serotype Dublin translocated in greater numbers than serotype Gallinarum. This result correlates positively with the ability of serotype Dublin, but not serotype Gallinarum, to induce disease in cattle. Thus, it appears that the mesenteric lymph nodes play an essential role in the containment of serotype Gallinarum translocation from the intestinal mucosa to systemic tissues.
In this study, we have investigated the nature of host-pathogen interactions during the early phases of infection that might influence the development of systemic salmonellosis. Survival within cells of the reticuloendothelial system is believed to be a key stage in Salmonella pathogenesis (5, 16, 21, 30). Furthermore, there is some evidence to suggest that the ability to survive within such cells contributes to the host-specific phenotype in vitro (2, 23, 31, 37, 47) and in vivo (5). The situation in vivo is clearly much more complicated, as serotype Typhimurium has been shown to kill murine hepatic macrophages (36). Other studies using avian (20), murine (35), or porcine (53) macrophages infected with strains of defined virulence demonstrate no such correlation between bacterial persistence within macrophages and host specificity. Furthermore, comparing the relative persistence of serotypes Dublin and Gallinarum in bovine macrophages is difficult for two reasons. First, alveolar macrophages from this host are susceptible to Salmonella-induced cell lysis (50). Second, the difference between motile and nonmotile serotypes, for example, serotypes Gallinarum and Pullorum, has a profound impact on their ability to interact with macrophages in vitro, thereby making results from such studies difficult to interpret (20, 55).
The persistence of serotype Dublin was significantly greater than that of serotype Gallinarum in the intestinal mucosa both following oral inoculation and 11 h after the infection of ligated ileal loops. Furthermore, only serotype Dublin, and not serotype Gallinarum, persisted at systemic sites following i.v. inoculation. These differences will likely have a major effect on the ability of the bacteria to spread from mucosa to lymph nodes, from lymph nodes to systemic sites, and from systemic sites back to the intestine (reseeding). Factors affecting bacterial persistence include the ability to acquire nutrients, the avoidance of the host's bactericidal mechanisms, and the ability to proliferate within the host. The balance among such aspects of pathogenesis therefore appears to be crucial in determining Salmonella serotype-host specificity in calves. The importance of bacterial persistence for the virulence of serotype Dublin in cattle has been demonstrated by the analysis of isogenic Salmonella pathogenicity island 2 mutants (8). Disruption of type three secretion system 2 blocked the persistence of serotype Dublin in bovine intestines and almost completely attenuated serotype Dublin infection in cattle. Type three secretion system 2-secreted effector proteins have been shown to influence Salmonella net growth by both promoting bacterial replication (38) and blocking the killing of bacteria by macrophages (45). Clearly, identification of the factors contributing to the increased persistence of serotype Dublin compared to serotype Gallinarum will provide greater insight into the molecular basis of Salmonella host specificity. However, bacterial persistence in niches other than macrophages may also be of fundamental importance in determining the outcome of infection. Future work should concentrate on characterizing how, and with which cells, Salmonella interacts in vivo, which will allow the design of more biologically relevant in vitro assays. Only then can dissection of the molecular mechanisms involved in persistence of different serotypes in vivo begin.
Acknowledgments
We thank Bryan Charleston for expert guidance in developing and performing the cannulation technique and Jennie Bispham and June Campbell for help with the processing of samples derived from the cannulation experiments.
This work was supported by DEFRA grant OZ0315 and BBSRC grants 201/510274 and 201/D10261 and by an Intervet studentship for S.M.P.
Editor: B. B. Finlay
REFERENCES
- 1.Allison, M. J., H. P. Dalton, M. R. Escobar, and C. J. Martin. 1969. Salmonella choleraesuis infections in man: a report of 19 cases and a critical literature review. South. Med. J. 62:593-596. [PubMed] [Google Scholar]
- 2.Alpuche-Aranda, C. M., E. P. Berthiaume, B. Mock, J. A. Swanson, and S. I. Miller. 1995. Spacious phagosome formation within mouse macrophages correlates with Salmonella serotype pathogenicity and host susceptibility. Infect. Immun. 63:4456-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin, I. I., G. R. Douce, M. P. Osborne, and J. Stephen. 1994. Quantitative studies of invasion of rabbit ileal mucosa by Salmonella typhimurium strains which differ in virulence in a model of gastroenteritis. Infect. Immun. 62:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baird, G. D., E. J. Manning, and P. W. Jones. 1985. Evidence for related virulence sequences in plasmids of Salmonella dublin and Salmonella typhimurium. J. Gen. Microbiol. 131:1815-1823. [DOI] [PubMed] [Google Scholar]
- 5.Barrow, P. A., M. B. Huggins, and M. A. Lovell. 1994. Host specificity of Salmonella infection in chickens and mice is expressed in vivo primarily at the level of the reticuloendothelial system. Infect. Immun. 62:4602-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baskerville, A., and C. Dow. 1973. Pathology of experimental pneumonia in pigs produced by Salmonella choleraesuis. J. Comp. Pathol. 83:207-215. [DOI] [PubMed] [Google Scholar]
- 7.Baumler, A. J., R. M. Tsolis, T. A. Ficht, and L. G. Adams. 1998. Evolution of host adaptation in Salmonella enterica. Infect. Immun. 66:4579-4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bispham, J., B. N. Tripathi, P. R. Watson, and T. S. Wallis. 2001. Salmonella pathogenicity island 2 influences both systemic salmonellosis and Salmonella-induced enteritis in calves. Infect. Immun. 69:367-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolton, A. J., M. P. Osborne, T. S. Wallis, and J. Stephen. 1999. Interaction of Salmonella choleraesuis, Salmonella dublin and Salmonella typhimurium with porcine and bovine terminal ileum in vivo. Microbiology 145:2431-2441. [DOI] [PubMed] [Google Scholar]
- 10.Buxton, A. 1957. Salmonellosis in animals. Commonwealth Agriculture Bureaux, Farnham Royal, England.
- 11.Carlson, G. P., and J. J. Kaneko. 1973. Isolation of leukocytes from bovine peripheral blood. Proc. Soc. Exp. Biol. Med. 142:853-856. [DOI] [PubMed] [Google Scholar]
- 12.Christensen, J. P., J. E. Olsen, H. C. Hansen, and M. Bisgaard. 1992. Characterization of Salmonella enterica serovar Gallinarum and Pullorum by plasmid profiling and biochemical analysis. Avian Pathol. 21:461-470. [DOI] [PubMed] [Google Scholar]
- 13.Colombo, M. M., G. Leori, S. Rubino, A. Barbato, and P. Cappuccinelli. 1992. Phenotypic features and molecular characterization of plasmids in S. abortusovis. J. Gen. Microbiol. 138:725-731. [Google Scholar]
- 14.Everest, P., J. Wain, M. Roberts, G. Rook, and G. Dougan. 2001. The molecular mechanisms of severe typhoid fever. Trends Microbiol. 9:316-320. [DOI] [PubMed] [Google Scholar]
- 15.Fang, F. C., and J. Fierer. 1991. Human infection with Salmonella dublin. Medicine (Baltimore) 70:198-207. [DOI] [PubMed] [Google Scholar]
- 16.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray, J. T., P. J. Fedorka-Cray, T. J. Stabel, and M. R. Ackermann. 1995. Influence of inoculation route on the carrier state of Salmonella choleraesuis in swine. Vet. Microbiol. 47:43-59. [DOI] [PubMed] [Google Scholar]
- 18.Gupta, B. R., and J. C. Verma. 1993. Monograph on animal salmonellosis. Veterinary Research Institute, Bareilly, India.
- 19.Hall, G. A., and P. W. Jones. 1976. An experimental study of Salmonella dublin abortion in cattle. Br. Vet. J. 132:60-65. [DOI] [PubMed] [Google Scholar]
- 20.Henderson, S. C., D. I. Bounous, and M. D. Lee. 1999. Early events in the pathogenesis of avian salmonellosis. Infect. Immun. 67:3580-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hensel, M., J. E. Shea, S. R. Waterman, R. Mundy, T. Nikolaus, G. Banks, A. Vazquez-Torres, C. Gleeson, F. C. Fang, and D. W. Holden. 1998. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 30:163-174. [DOI] [PubMed] [Google Scholar]
- 22.Hornick, R. B., S. E. Greisman, T. E. Woodward, H. L. DuPont, A. T. Dawkins, and M. J. Snyder. 1970. Typhoid fever: pathogenesis and immunologic control. N. Engl. J. Med. 283:686-691. [DOI] [PubMed] [Google Scholar]
- 23.Ishibashi, Y., and T. Arai. 1996. A possible mechanism for host-specific pathogenesis of Salmonella serovars. Microb. Pathog. 21:435-446. [DOI] [PubMed] [Google Scholar]
- 24.Jack, E. J. 1968. Salmonella abortusovis: an atypical Salmonella. Vet. Rec. 82:558-561. [Google Scholar]
- 25.Jones, P. W. 1975. The effect of storage in slurry on the virulence of Salmonella dublin. J. Hyg. 74:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, P. W., P. Collins, and M. M. Aitken. 1988. Passive protection of calves against experimental infection with Salmonella typhimurium. Vet. Rec. 123:536-541. [DOI] [PubMed] [Google Scholar]
- 27.Kingsley, R. A., and A. J. Baumler. 2000. Host adaptation and the emergence of infectious disease: the Salmonella paradigm. Mol. Microbiol. 36:1006-1014. [DOI] [PubMed] [Google Scholar]
- 28.Lantier, F., P. Pardon, and J. Marly. 1983. Immunogenicity of a low-virulence vaccinal strain against Salmonella abortusovis infection in mice. Infect. Immun. 40:601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lax, A. J., P. A. Barrow, P. W. Jones, and T. S. Wallis. 1995. Current perspectives in salmonellosis. Br. Vet. J. 151:351-377. [DOI] [PubMed] [Google Scholar]
- 30.Libby, S. J., W. Goebel, A. Ludwig, N. Buchmeier, F. Bowe, F. C. Fang, D. G. Guiney, J. G. Songer, and F. Heffron. 1994. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc. Natl. Acad. Sci. USA 91:489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lissner, C. R., D. L. Weinstein, and A. D. O'Brien. 1985. Mouse chromosome 1 Ity locus regulates microbicidal activity of isolated peritoneal macrophages against a diverse group of intracellular and extracellular bacteria. J. Immunol. 135:544-547. [PubMed] [Google Scholar]
- 32.McCormick, B. A., P. M. Hofman, J. Kim, D. K. Carnes, S. I. Miller, and J. L. Madara. 1995. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J. Cell Biol. 131:1599-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormick, B. A., S. I. Miller, D. Carnes, and J. L. Madara. 1995. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect. Immun. 63:2302-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsons, K. R., C. J. Howard, B. V. Jones, and P. Sopp. 1989. Investigation of bovine gut associated lymphoid tissue (GALT) using monoclonal antibodies against bovine lymphocytes. Vet. Pathol. 26:396-408. [DOI] [PubMed] [Google Scholar]
- 35.Pascopella, L., B. Raupach, N. Ghori, D. Monack, S. Falkow, and P. L. Small. 1995. Host restriction phenotypes of Salmonella typhi and Salmonella gallinarum. Infect. Immun. 63:4329-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richter-Dahlfors, A., A. M. Buchan, and B. B. Finlay. 1997. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 186:569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwan, W. R., and D. J. Kopecko. 1997. Serovar specific differences in Salmonella survival within macrophage cells. Adv. Exp. Med. Biol. 412:277-278. [DOI] [PubMed] [Google Scholar]
- 38.Shea, J. E., C. R. Beuzon, C. Gleeson, R. Mundy, and D. W. Holden. 1999. Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infect. Immun. 67:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shivaprased, H. L. 2000. Fowl typhoid and pullorum disease. Rev. Sci. Tech. 19:405-424. [DOI] [PubMed] [Google Scholar]
- 40.Smith, H. W., and S. Halls. 1968. The simultaneous oral administration of Salmonella dublin, S. typhimurium and S. choleraesuis to calves and other animals. J. Med. Microbiol. 1:203-209. [DOI] [PubMed] [Google Scholar]
- 41.Sojka, W. J., and H. I. Field. 1970. Salmonellosis in England and Wales 1958-1967. Vet. Bull. 40:515-531. [Google Scholar]
- 42.Sojka, W. J., C. Wray, and J. Shreeve. 1977. Incidence of Salmonella infection in animals in England and Wales, 1968-1974. J. Hyg. 78:43-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uzzau, S., D. J. Brown, T. Wallis, S. Rubino, G. Leori, S. Bernard, J. Casadesus, D. J. Platt, and J. E. Olsen. 2000. Host adapted serotypes of Salmonella enterica. Epidemiol. Infect. 125:229-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uzzau, S., G. S. Leori, V. Petruzzi, P. R. Watson, G. Schianchi, D. Bacciu, V. Mazzarello, T. S. Wallis, and S. Rubino. 2001. Salmonella serotype-host specificity does not correlate with the magnitude of intestinal invasion in sheep. Infect. Immun. 69:3092-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 46.Veterinary Laboratories Agency. 1978-1999. Salmonella in livestock production. Veterinary Laboratories Agency, Ministry of Agriculture, Fisheries and Food, Addlestone, United Kingdom.
- 47.Vladoianu, I. R., H. R. Chang, and J. C. Pechere. 1990. Expression of host resistance to Salmonella typhi and Salmonella typhimurium: bacterial survival within macrophages of murine and human origin. Microb. Pathog. 8:83-90. [DOI] [PubMed] [Google Scholar]
- 48.Wallis, T. S., S. M. Paulin, J. S. Plested, P. R. Watson, and P. W. Jones. 1995. The Salmonella dublin virulence plasmid mediates systemic but not enteric phases of salmonellosis in cattle. Infect. Immun. 63:2755-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson, P. R., E. E. Galyov, S. M. Paulin, P. W. Jones, and T. S. Wallis. 1998. Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect. Immun. 66:1432-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watson, P. R., A. V. Gautier, S. M. Paulin, A. P. Bland, P. W. Jones, and T. S. Wallis. 2000. Salmonella enterica serovars Typhimurium and Dublin can lyse macrophages by a mechanism distinct from apoptosis. Infect. Immun. 68:3744-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watson, P. R., S. M. Paulin, A. P. Bland, P. W. Jones, and T. S. Wallis. 1995. Characterization of intestinal invasion by Salmonella typhimurium and Salmonella dublin and effect of a mutation in the invH gene. Infect. Immun. 63:2743-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watson, P. R., S. M. Paulin, A. P. Bland, S. J. Libby, P. W. Jones, and T. S. Wallis. 1999. Differential regulation of enteric and systemic salmonellosis by slyA. Infect. Immun. 67:4950-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson, P. R., S. M. Paulin, P. W. Jones, and T. S. Wallis. 2000. Interaction of Salmonella serotypes with porcine macrophages in vitro does not correlate with virulence. Microbiology 146:1639-1649. [DOI] [PubMed] [Google Scholar]
- 54.Williams Smith, H. 1955. Observations on experimental fowl typhoid. J. Comp. Fowl Typhoid 65:37-54. [DOI] [PubMed] [Google Scholar]
- 55.Wilson, R. L., J. Elthon, S. Clegg, and B. D. Jones. 2000. Salmonella enterica serovars Gallinarum and Pullorum expressing Salmonella enterica serovar Typhimurium type 1 fimbriae exhibit increased invasiveness for mammalian cells. Infect. Immun. 68:4782-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]