Abstract
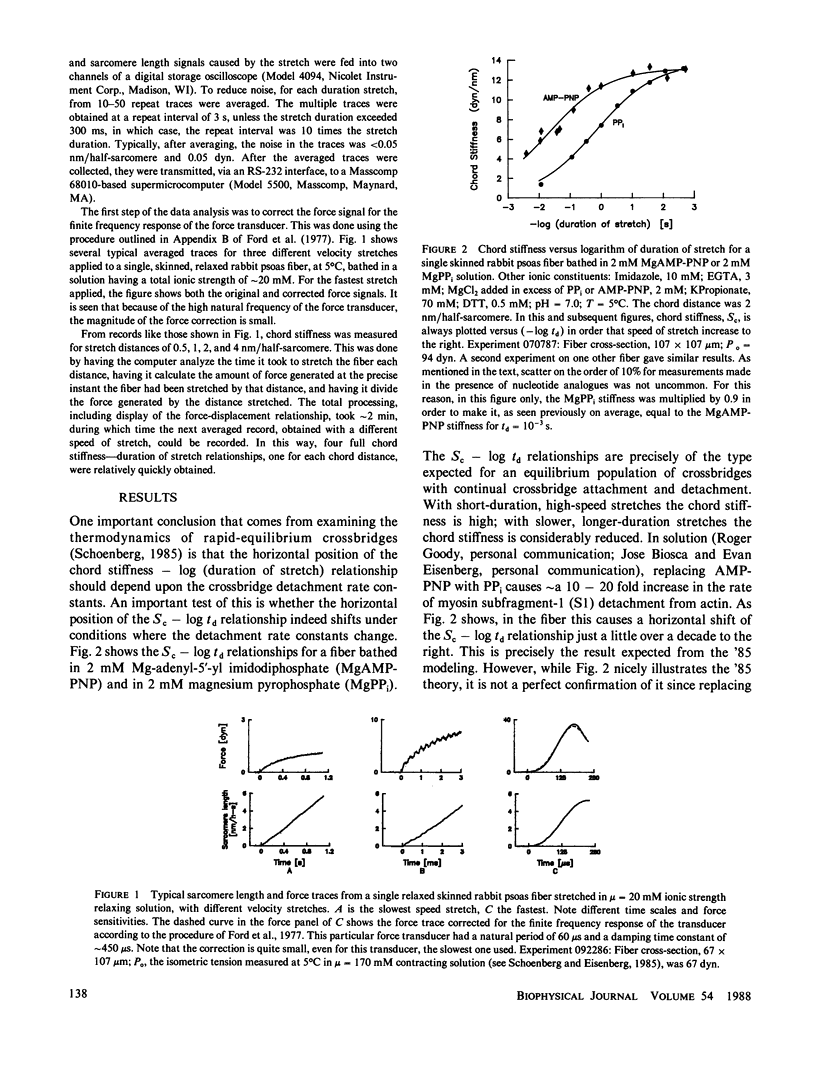
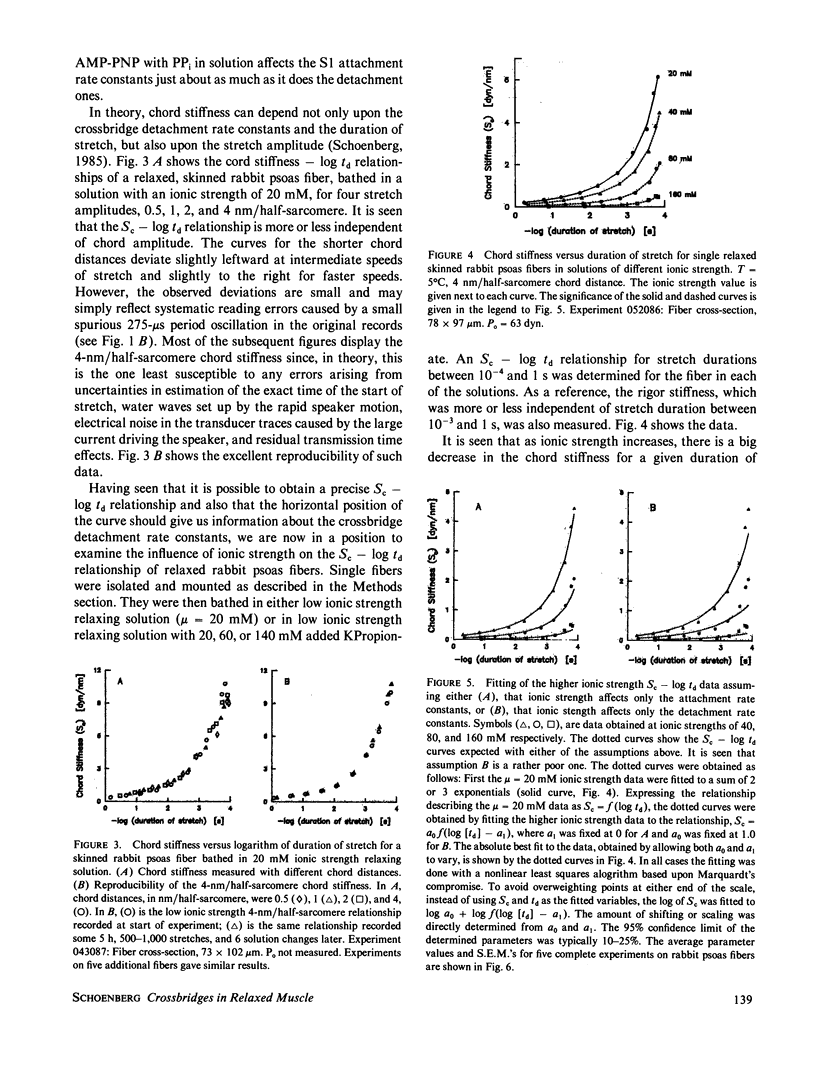
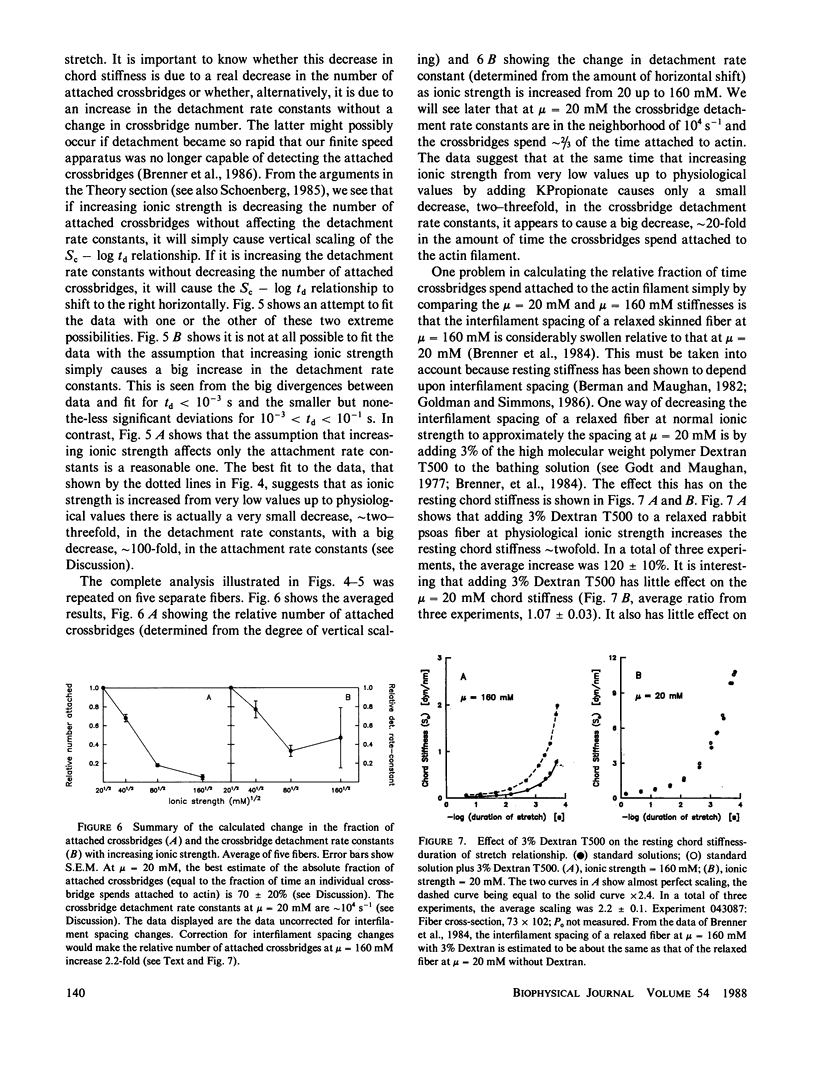
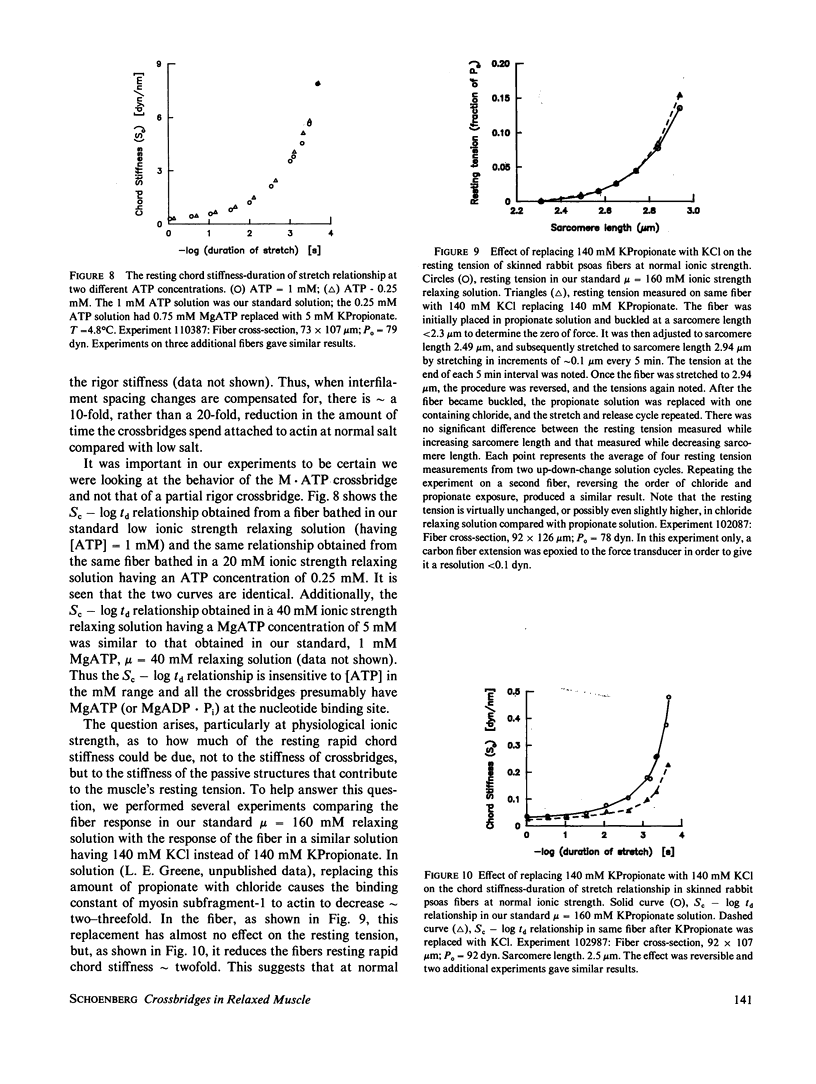
In the presence of ATP and absence of Ca2+, muscle crossbridges have either MgATP or MgADP.Pi bound at the active site (S. B. Marston and R. T. Tregear, Nature [Lond.], 235:22:1972). The behavior of these myosin adenosine triphosphate (M.ATP) crossbridges, both in relaxed skinned rabbit psoas and frog semitendinosus fibers, was analyzed. At very low ionic strength, T = 5 degrees C, mu = 20 mM, these crossbridges spend a large fraction of the time attached to actin. In rabbit, the attachment rate constants at low salt are 10(4) - 10(5) s-1, and the detachment rate constants are approximately 10(4) s-1. When ionic strength is increased up to physiological values by addition of 140 mM potassium propionate, the major effect is a weakening of the crossbridge binding constant approximately 30-40-fold. This effect occurs because of a large decrease, approximately 100-fold, in the crossbridge attachment rate constants. The detachment rate constants decrease only 2-3-fold. The effect of ionic strength on crossbridge binding in the fiber is very similar to the effect of ionic strength on the binding of myosin subfragment-1 to unregulated actin in solution. Thus, the effect of increasing ionic strength in fibers appears to be a direct effect on crossbridge binding rather than an effect on troponin-tropomyosin. The finding that crossbridges with ATP bound at the active site can and do attach to actin over a wide range of ionic strengths strongly suggests that troponin-tropomyosin keeps a muscle relaxed by blocking a step subsequent to crossbridge attachment. Thus, rather than troponin-tropomyosin serving to keep a muscle relaxed by inhibiting attachment, it seems quite possible that the main way in which troponin-tropomyosin regulates muscle activity is by preventing the weakly-binding relaxed crossbridges from going on through the crossbridge cycle into more strongly-binding states.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagshaw C. R., Trentham D. R. The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J. 1974 Aug;141(2):331–349. doi: 10.1042/bj1410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M. R., Maughan D. W. Axial elastic modulus as a function of relative fiber width in relaxed skinned skeletal muscle fibers. Pflugers Arch. 1982 Mar;393(1):99–103. doi: 10.1007/BF00582400. [DOI] [PubMed] [Google Scholar]
- Brenner B., Chalovich J. M., Greene L. E., Eisenberg E., Schoenberg M. Stiffness of skinned rabbit psoas fibers in MgATP and MgPPi solution. Biophys J. 1986 Oct;50(4):685–691. doi: 10.1016/S0006-3495(86)83509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Sarcomeric domain organization within single skinned rabbit psoas fibers and its effects on laser light diffraction patterns. Biophys J. 1985 Dec;48(6):967–982. doi: 10.1016/S0006-3495(85)83860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Podolsky R. J. X-ray diffraction evidence for cross-bridge formation in relaxed muscle fibers at various ionic strengths. Biophys J. 1984 Sep;46(3):299–306. doi: 10.1016/S0006-3495(84)84026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. The effect of troponin-tropomyosin on the binding of heavy meromyosin to actin in the presence of ATP. J Biol Chem. 1986 Apr 15;261(11):5088–5093. [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Franks K. All myosin heads form bonds with actin in rigor rabbit skeletal muscle. Biochemistry. 1980 May 13;19(10):2265–2269. doi: 10.1021/bi00551a042. [DOI] [PubMed] [Google Scholar]
- Craig R., Greene L. E., Eisenberg E. Structure of the actin-myosin complex in the presence of ATP. Proc Natl Acad Sci U S A. 1985 May;82(10):3247–3251. doi: 10.1073/pnas.82.10.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood A. B., Wood D. S., Bock K. L., Sorenson M. M. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell. 1979;11(3):553–566. doi: 10.1016/0040-8166(79)90062-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Greene L. E. The relation of muscle biochemistry to muscle physiology. Annu Rev Physiol. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves M. A., Jeffries T. E., Millar N. C. ATP-induced dissociation of rabbit skeletal actomyosin subfragment 1. Characterization of an isomerization of the ternary acto-S1-ATP complex. Biochemistry. 1986 Dec 30;25(26):8454–8458. doi: 10.1021/bi00374a020. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. Swelling of skinned muscle fibers of the frog. Experimental observations. Biophys J. 1977 Aug;19(2):103–116. doi: 10.1016/S0006-3495(77)85573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5'-triphosphate. J Physiol. 1984 Sep;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E. Measurement of sarcomere shortening in skinned fibers from frog muscle by white light diffraction. Biophys J. 1987 Jul;52(1):57–68. doi: 10.1016/S0006-3495(87)83188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Active and rigor muscle stiffness [proceedings]. J Physiol. 1977 Jul;269(1):55P–57P. [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. The stiffness of frog skinned muscle fibres at altered lateral filament spacing. J Physiol. 1986 Sep;378:175–194. doi: 10.1113/jphysiol.1986.sp016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Dissociation of the actin.subfragment 1 complex by adenyl-5'-yl imidodiphosphate, ADP, and PPi. J Biol Chem. 1980 Jan 25;255(2):543–548. [PubMed] [Google Scholar]
- Greene L. E., Sellers J. R., Eisenberg E., Adelstein R. S. Binding of gizzard smooth muscle myosin subfragment 1 to actin in the presence and absence of adenosine 5'-triphosphate. Biochemistry. 1983 Feb 1;22(3):530–535. doi: 10.1021/bi00272a002. [DOI] [PubMed] [Google Scholar]
- Gulati J. Magnesium ion-dependent contraction of skinned frog muscle fibers in calcium-free solution. Biophys J. 1983 Oct;44(1):113–121. doi: 10.1016/S0006-3495(83)84283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen P., Sten-Knudsen O. The dependence of the short-range elasticity on sarcomere length in resting isolated frog muscle fibres. Acta Physiol Scand. 1981 Jun;112(2):113–120. doi: 10.1111/j.1748-1716.1981.tb06793.x. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Trentham D. R. Relationships between chemical and mechanical events during muscular contraction. Annu Rev Biophys Biophys Chem. 1986;15:119–161. doi: 10.1146/annurev.bb.15.060186.001003. [DOI] [PubMed] [Google Scholar]
- Highsmith S. Interactions of the actin and nucleotide binding sites on myosin subfragment 1. J Biol Chem. 1976 Oct 25;251(20):6170–6172. [PubMed] [Google Scholar]
- Hill D. K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol. 1968 Dec;199(3):637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell S. J., Harrington W. F. Measurement of the fraction of myosin heads bound to actin in rabbit skeletal myofibrils in rigor. J Mol Biol. 1981 Jul 15;149(4):659–674. doi: 10.1016/0022-2836(81)90352-1. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Lännergren J. The effect of low-level activation on the mechanical properties of isolated frog muscle fibers. J Gen Physiol. 1971 Aug;58(2):145–162. doi: 10.1085/jgp.58.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Tregear R. T. Evidence for a complex between myosin and ADP in relaxed muscle fibres. Nat New Biol. 1972 Jan 5;235(53):23–24. doi: 10.1038/newbio235023a0. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Tregear R. T., Rodger C. D., Clarke M. L. Coupling between the enzymatic site of myosin and the mechanical output of muscle. J Mol Biol. 1979 Feb 25;128(2):111–126. doi: 10.1016/0022-2836(79)90121-9. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Podolsky R. J. X-ray evidence for two structural states of the actomyosin cross-bridge in muscle fibers. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2364–2368. doi: 10.1073/pnas.81.8.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry D. A., Squire J. M. Structural role of tropomyosin in muscle regulation: analysis of the x-ray diffraction patterns from relaxed and contracting muscles. J Mol Biol. 1973 Mar 25;75(1):33–55. doi: 10.1016/0022-2836(73)90527-5. [DOI] [PubMed] [Google Scholar]
- Rosenfeld S. S., Taylor E. W. The mechanism of regulation of actomyosin subfragment 1 ATPase. J Biol Chem. 1987 Jul 25;262(21):9984–9993. [PubMed] [Google Scholar]
- Rüdel R., Zite-Ferenczy F. Do laser diffraction studies on striated muscle indicate stepwise sarcomere shortening? Nature. 1979 Apr 5;278(5704):573–575. doi: 10.1038/278573a0. [DOI] [PubMed] [Google Scholar]
- Schoenberg M., Brenner B., Chalovich J. M., Greene L. E., Eisenberg E. Cross-bridge attachment in relaxed muscle. Adv Exp Med Biol. 1984;170:269–284. doi: 10.1007/978-1-4684-4703-3_24. [DOI] [PubMed] [Google Scholar]
- Schoenberg M., Eisenberg E. Muscle cross-bridge kinetics in rigor and in the presence of ATP analogues. Biophys J. 1985 Dec;48(6):863–871. doi: 10.1016/S0006-3495(85)83847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M. Equilibrium muscle cross-bridge behavior. Theoretical considerations. Biophys J. 1985 Sep;48(3):467–475. doi: 10.1016/S0006-3495(85)83802-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M. The kinetics of weakly- and strongly-binding crossbridges: implications for contraction and relaxation. Adv Exp Med Biol. 1988;226:189–202. [PubMed] [Google Scholar]
- Schoenberg M., Wells J. B., Podolsky R. J. Muscle compliance and the longitudinal transmission of mechanical impulses. J Gen Physiol. 1974 Dec;64(6):623–642. doi: 10.1085/jgp.64.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M., Wells J. B. Stiffness, force, and sarcomere shortening during a twitch in frog semitendinosus muscle bundles. Biophys J. 1984 Feb;45(2):389–397. doi: 10.1016/S0006-3495(84)84163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegman M. J., Butler T. M., Mooers S. U., Davies R. E. Crossbridge attachment, resistance to stretch, and viscoelasticity in resting mammalian smooth muscle. Science. 1976 Jan 30;191(4225):383–385. doi: 10.1126/science.128819. [DOI] [PubMed] [Google Scholar]
- Stein L. A., Schwarz R. P., Jr, Chock P. B., Eisenberg E. Mechanism of actomyosin adenosine triphosphatase. Evidence that adenosine 5'-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry. 1979 Sep 4;18(18):3895–3909. doi: 10.1021/bi00585a009. [DOI] [PubMed] [Google Scholar]
- Sundell C. L., Goldman Y. E., Peachey L. D. Fine structure in near-field and far-field laser diffraction patterns from skeletal muscle fibers. Biophys J. 1986 Feb;49(2):521–530. doi: 10.1016/S0006-3495(86)83662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner P. D. Effect of skeletal muscle myosin light chain 2 on the Ca2+-sensitive interaction of myosin and heavy meromyosin with regulated actin. Biochemistry. 1984 Dec 4;23(25):5950–5956. doi: 10.1021/bi00320a010. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Giniger E. Calcium-sensitive binding of heavy meromyosin to regulated actin in the presence of ATP. J Biol Chem. 1981 Dec 25;256(24):12647–12650. [PubMed] [Google Scholar]
- Wakabayashi K., Namba K., Mitsui T. Configurations of myosin heads in the crab striated muscle as studied by X-ray diffraction. Adv Exp Med Biol. 1984;170:237–250. doi: 10.1007/978-1-4684-4703-3_21. [DOI] [PubMed] [Google Scholar]
- Xu S. G., Kress M., Huxley H. E. X-ray diffraction studies of the structural state of crossbridges in skinned frog sartorius muscle at low ionic strength. J Muscle Res Cell Motil. 1987 Feb;8(1):39–54. doi: 10.1007/BF01767263. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Herzig J. W. Series elastic properties of skinned muscle fibres in contraction and rigor. Pflugers Arch. 1978 Jan 31;373(1):21–24. doi: 10.1007/BF00581144. [DOI] [PubMed] [Google Scholar]


