Abstract
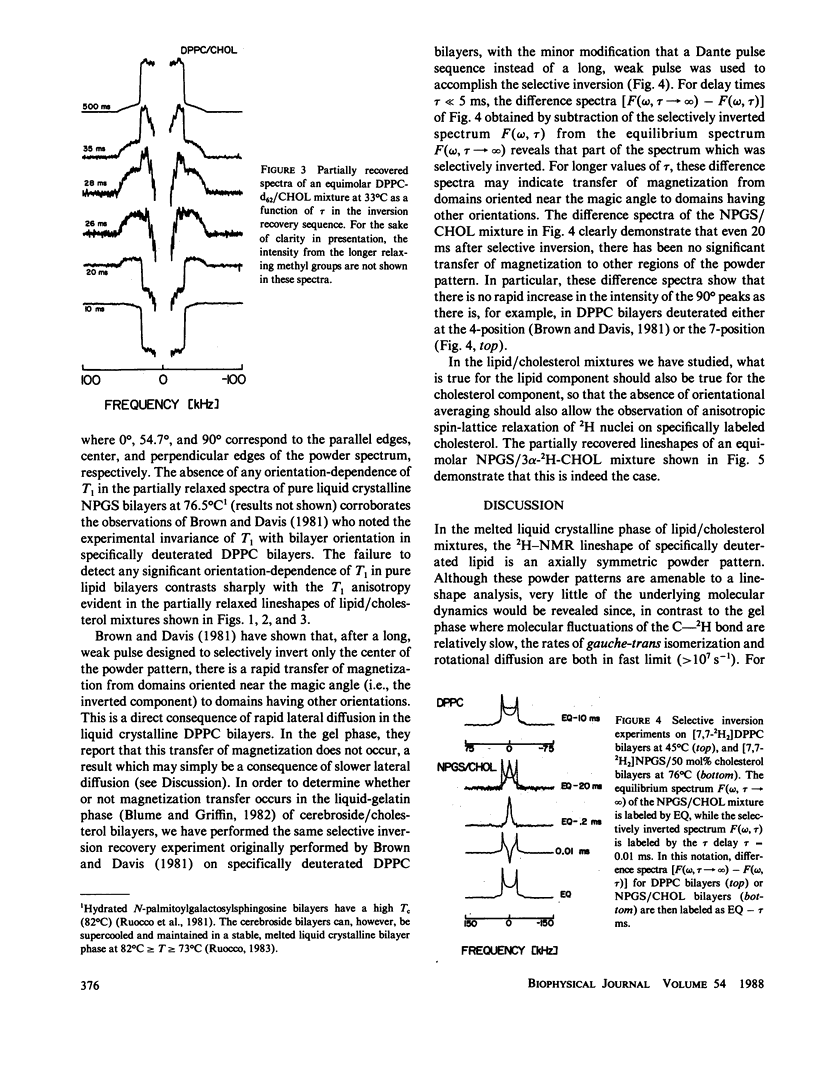
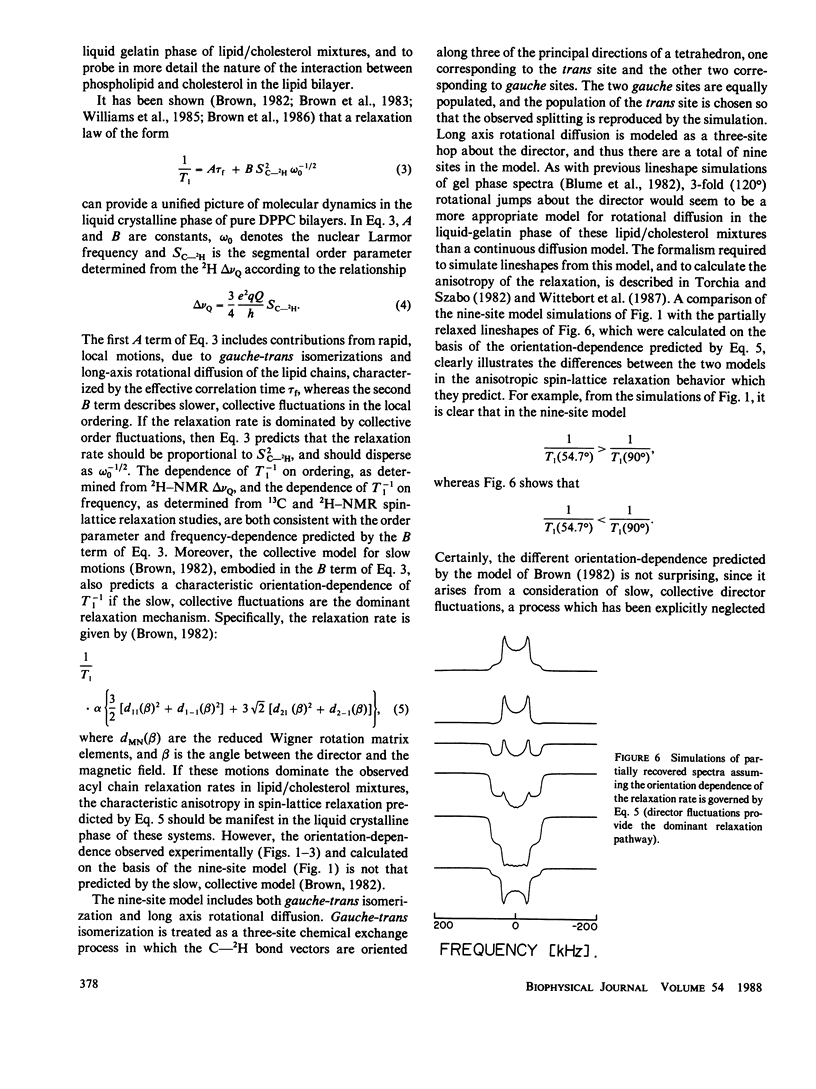
The axially symmetric powder pattern 2H-nuclear magnetic resonance (NMR) lineshapes observed in the liquid crystalline phase of pure lipid or lipid/cholesterol bilayers are essentially invariant to temperature, or, equivalently, to variations in the correlation times characterizing C-2H bond reorientations. In either of these melted phases, where correlation times for C-2H bond motions are shorter than 10(-7) s, information on the molecular dynamics of the saturated hydrocarbon chain would be difficult to obtain using lineshape analyses alone, and one must resort to other methods, such as the measurement of 2H spin-lattice relaxation rates, in order to obtain dynamic information. In pure lipid bilayers, the full power of the spin-lattice relaxation technique has yet to be realized, since an important piece of information, namely the orientation dependence of the 2H spin-lattice relaxation rates is usually lost due to orientational averaging of T1 by rapid lateral diffusion. Under more favorable circumstances, such as those encountered in the lipid/cholesterol mixtures of this study, the effects of orientational averaging by lateral diffusion are nullified, due to either a marked reduction (by at least an order of magnitude) in the diffusion rate, or a marked increase in the radii of curvature of the liposomes. In either case, the angular dependence of 2H spin-lattice relaxation is accessible to experimental study, and can be used to test models of molecular dynamics in these systems. Simulations of the partially recovered lineshapes indicate that the observed T1 anisotropies are consistent with large amplitude molecular reorientation of the C-2H bond among a finite number of sites. Furthermore, from the observed orientation dependence of the 2H spin-lattice relaxation rates, we conclude that order director fluctuations cannot provide the dominant relaxation pathway for acyl chain deuterons.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akutsu H., Seelig J. Interaction of metal ions with phosphatidylcholine bilayer membranes. Biochemistry. 1981 Dec 22;20(26):7366–7373. doi: 10.1021/bi00529a007. [DOI] [PubMed] [Google Scholar]
- Blume A., Griffin R. G. Carbon-13 and deuterium nuclear magnetic resonance study of the interaction of cholesterol with phosphatidylethanolamine. Biochemistry. 1982 Nov 23;21(24):6230–6242. doi: 10.1021/bi00267a031. [DOI] [PubMed] [Google Scholar]
- Blume A., Rice D. M., Wittebort R. J., Griffin R. G. Molecular dynamics and conformation in the gel and liquid-crystalline phases of phosphatidylethanolamine bilayers. Biochemistry. 1982 Nov 23;21(24):6220–6230. doi: 10.1021/bi00267a030. [DOI] [PubMed] [Google Scholar]
- Brown M. F., Ribeiro A. A., Williams G. D. New view of lipid bilayer dynamics from 2H and 13C NMR relaxation time measurements. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4325–4329. doi: 10.1073/pnas.80.14.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar D. L., Kirschner D. A. Myelin membrane structure at 10 A resolution. Nat New Biol. 1971 May 12;231(19):46–52. doi: 10.1038/newbio231046a0. [DOI] [PubMed] [Google Scholar]
- DasGupta S. K., Rice D. M., Griffin R. G. Synthesis of isotopically labeled saturated fatty acids. J Lipid Res. 1982 Jan;23(1):197–200. [PubMed] [Google Scholar]
- Gally H. U., Seelig A., Seelig J. Cholesterol-induced rod-like motion of fatty acyl chains in lipid bilayers a deuterium magnetic resonance study. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(10):1447–1450. [PubMed] [Google Scholar]
- Griffin R. G. Solid state nuclear magnetic resonance of lipid bilayers. Methods Enzymol. 1981;72:108–174. doi: 10.1016/s0076-6879(81)72010-x. [DOI] [PubMed] [Google Scholar]
- Haberkorn R. A., Griffin R. G., Meadows M. D., Oldfield E. Deuterium nuclear magnetic resonance investigation of the dipalmitoyl lecithin-cholesterol-water system. J Am Chem Soc. 1977 Oct 26;99(22):7353–7355. doi: 10.1021/ja00464a043. [DOI] [PubMed] [Google Scholar]
- Hui S. W., He N. B. Molecular organization in cholesterol-lecithin bilayers by X-ray and electron diffraction measurements. Biochemistry. 1983 Mar 1;22(5):1159–1164. doi: 10.1021/bi00274a026. [DOI] [PubMed] [Google Scholar]
- Jacobs R., Oldfield E. Deuterium nuclear magnetic resonance investigation of dimyristoyllecithin--dipalmitoyllecithin and dimyristoyllecithin--cholesterol mixtures. Biochemistry. 1979 Jul 24;18(15):3280–3285. doi: 10.1021/bi00582a013. [DOI] [PubMed] [Google Scholar]
- Lecuyer H., Dervichian D. G. Structure of aqueous mixtures of lecithin and cholesterol. J Mol Biol. 1969 Oct 14;45(1):39–57. doi: 10.1016/0022-2836(69)90208-3. [DOI] [PubMed] [Google Scholar]
- Linington C., Rumsby M. G. Accessibility of galactosyl ceramides to probe reagents in central nervous system myelin. J Neurochem. 1980 Oct;35(4):983–992. doi: 10.1111/j.1471-4159.1980.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Linington C., Rumsby M. G. On the accessibility and localisation of cerebrosides in central nervous system myelin. Adv Exp Med Biol. 1978;100:263–273. doi: 10.1007/978-1-4684-2514-7_19. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim Biophys Acta. 1978 Oct 19;513(1):43–58. doi: 10.1016/0005-2736(78)90110-4. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Meadows M., Rice D., Jacobs R. Spectroscopic studies of specifically deuterium labeled membrane systems. Nuclear magnetic resonance investigation of the effects of cholesterol in model systems. Biochemistry. 1978 Jul 11;17(14):2727–2740. doi: 10.1021/bi00607a006. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruocco M. J., Atkinson D., Small D. M., Skarjune R. P., Oldfield E., Shipley G. G. X-ray diffraction and calorimetric study of anhydrous and hydrated N-palmitoylgalactosylsphingosine (cerebroside). Biochemistry. 1981 Oct 13;20(21):5957–5966. doi: 10.1021/bi00524a006. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Shipley G. G. Interaction of cholesterol with galactocerebroside and galactocerebroside-phosphatidylcholine bilayer membranes. Biophys J. 1984 Dec;46(6):695–707. doi: 10.1016/S0006-3495(84)84068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch D. J., Brown M. F., Jeffrey K. R. 14N NMR of lipid bilayers: effects of ions and anesthetics. Biochemistry. 1984 May 22;23(11):2412–2420. doi: 10.1021/bi00306a015. [DOI] [PubMed] [Google Scholar]
- Siminovitch D. J., Ruocco M. J., Makriyannis A., Griffin R. G. The effect of cholesterol on lipid dynamics and packing in diether phosphatidylcholine bilayers. X-ray diffraction and 2H-NMR study. Biochim Biophys Acta. 1987 Jul 23;901(2):191–200. doi: 10.1016/0005-2736(87)90115-5. [DOI] [PubMed] [Google Scholar]
- Tamm L. K., McConnell H. M. Supported phospholipid bilayers. Biophys J. 1985 Jan;47(1):105–113. doi: 10.1016/S0006-3495(85)83882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. G., Akiyama T., Saitô H., Smith I. C. Direct observation of the properties of cholesterol in membranes by deuterium NMR. Chem Phys Lipids. 1982 Dec;31(4):359–379. doi: 10.1016/0009-3084(82)90072-x. [DOI] [PubMed] [Google Scholar]


