Abstract
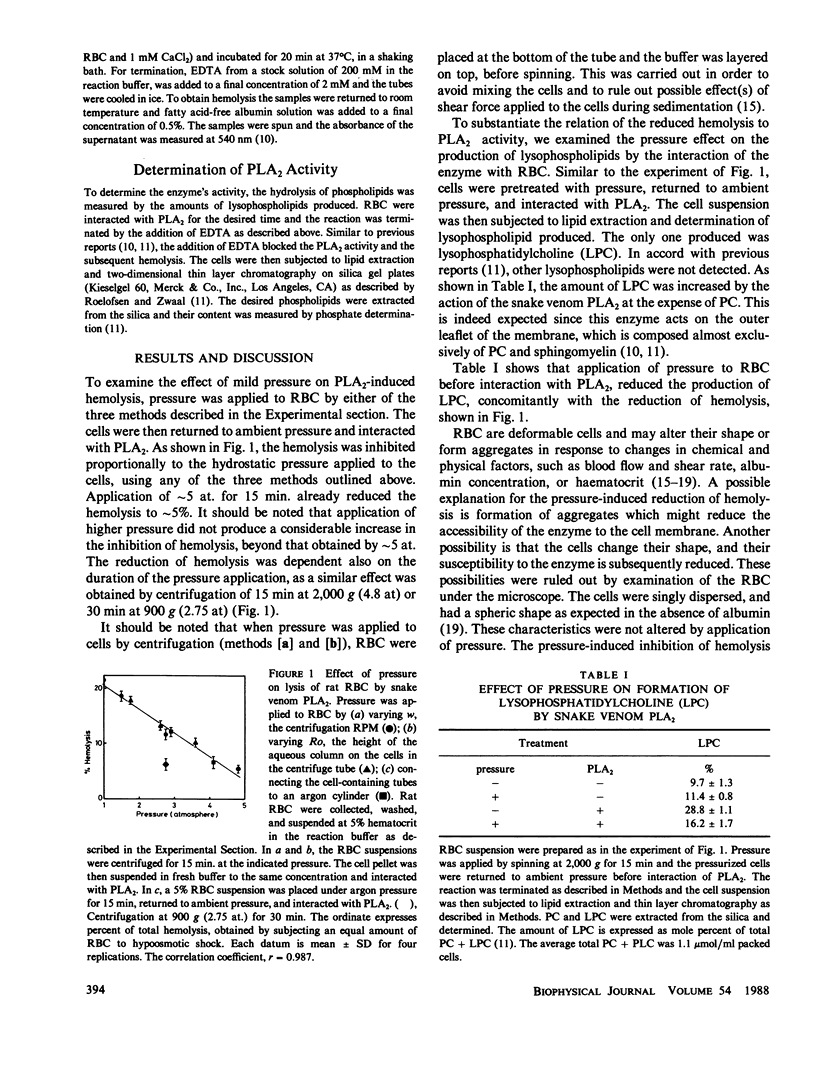
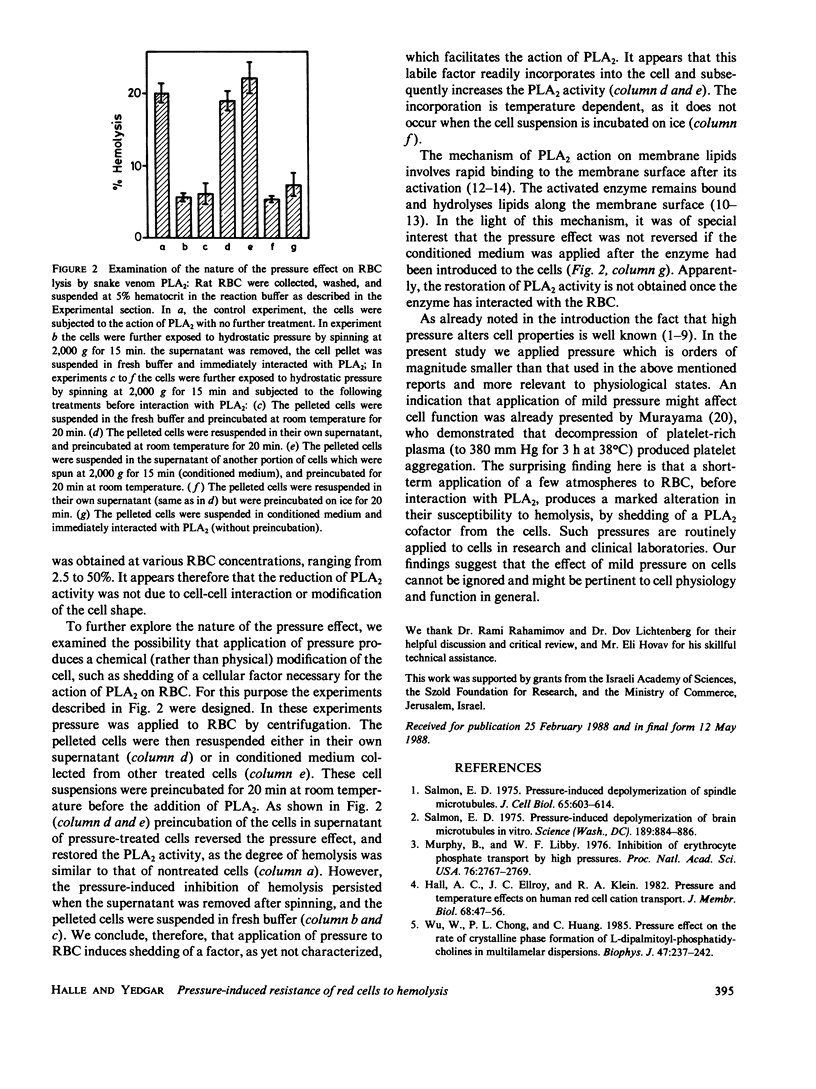
It is generally assumed that mild pressure of a few atmospheres, such as that applied to blood cells during routine centrifugation, does not affect cell function. The results of the present study refute this notion. To explore the effect of mild pressure on cell function we examined its effect on the susceptibility of red blood cells (RBC) to hemolysis by snake venom phospholipase A2 (PLA2). Rat RBC were subjected to pressure of up to five atmospheres, returned to ambient pressure and interacted with PLA2 to induce hemolysis. The hemolysis was markedly decreased with increasing the pressure applied before induction of hemolysis. Application of such a pressure induces the shedding of a chemical factor, as yet uncharacterized, which facilitates the action of PLA2 on RBC.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braganza L. F., Worcester D. L. Structural changes in lipid bilayers and biological membranes caused hydrostatic pressure. Biochemistry. 1986 Nov 18;25(23):7484–7488. doi: 10.1021/bi00371a034. [DOI] [PubMed] [Google Scholar]
- Chien S., Sung L. A., Simchon S., Lee M. M., Jan K. M., Skalak R. Energy balance in red cell interactions. Ann N Y Acad Sci. 1983;416:190–206. doi: 10.1111/j.1749-6632.1983.tb35189.x. [DOI] [PubMed] [Google Scholar]
- Deckmann M., Haimovitz R., Shinitzky M. Selective release of integral proteins from human erythrocyte membranes by hydrostatic pressure. Biochim Biophys Acta. 1985 Dec 5;821(2):334–340. doi: 10.1016/0005-2736(85)90103-8. [DOI] [PubMed] [Google Scholar]
- Dintenfass L. Execution of "ARC" experiment on space shuttle "Discovery" STS 51-C: some results on aggregation of red blood cells under zero gravity. Biorheology. 1986;23(4):331–347. doi: 10.3233/bir-1986-23403. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Parsegian V. A. Energetics of membrane deformation and adhesion in cell and vesicle aggregation. Ann N Y Acad Sci. 1983;416:13–33. doi: 10.1111/j.1749-6632.1983.tb35176.x. [DOI] [PubMed] [Google Scholar]
- Hall A. C., Ellory J. C., Klein R. A. Pressure and temperature effects on human red cell cation transport. J Membr Biol. 1982;68(1):47–56. doi: 10.1007/BF01872253. [DOI] [PubMed] [Google Scholar]
- Murayama M. Compression inhibits aggregation of human platelets under high hydraulic pressure. Thromb Res. 1987 Mar 15;45(6):729–738. doi: 10.1016/0049-3848(87)90083-1. [DOI] [PubMed] [Google Scholar]
- Murayama M. Ex vivo human platelet aggregation induced by decompression during reduced barometric pressure, hydrostatic, and hydrodynamic (Bernoulli) effect. Thromb Res. 1984 Mar 1;33(5):477–485. doi: 10.1016/0049-3848(84)90013-6. [DOI] [PubMed] [Google Scholar]
- Murphy R. B., Libby W. F. Inhibition of erythrocyte phosphate transport by high pressures. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2767–2769. doi: 10.1073/pnas.73.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfafferott C., Nash G. B., Meiselman H. J. Red blood cell deformation in shear flow. Effects of internal and external phase viscosity and of in vivo aging. Biophys J. 1985 May;47(5):695–704. doi: 10.1016/S0006-3495(85)83966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. F., Deems R. A., Dennis E. A. Dual role of interfacial phospholipid in phospholipase A2 catalysis. Proc Natl Acad Sci U S A. 1977 May;74(5):1950–1954. doi: 10.1073/pnas.74.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer C. A., Weber G., Daly T. J., Matthews K. S. Dissociation of the lactose repressor protein tetramer using high hydrostatic pressure. Biochemistry. 1986 Dec 16;25(25):8308–8315. doi: 10.1021/bi00373a027. [DOI] [PubMed] [Google Scholar]
- Salmon E. D. Pressure-induced depolymerization of brain microtubules in vitro. Science. 1975 Sep 12;189(4206):884–886. doi: 10.1126/science.1171523. [DOI] [PubMed] [Google Scholar]
- Salmon E. D. Pressure-induced depolymerization of spindle microtubules. I. Changes in birefringence and spindle length. J Cell Biol. 1975 Jun;65(3):603–614. doi: 10.1083/jcb.65.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. G., Chong P. L., Huang C. H. Pressure effect on the rate of crystalline phase formation of L-alpha-dipalmitoylphosphatidylcholines in multilamellar dispersions. Biophys J. 1985 Feb;47(2 Pt 1):237–242. doi: 10.1016/s0006-3495(85)83896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B., Colley C. M. Localization of red cell membrane constituents. Biochim Biophys Acta. 1973 Sep 10;300(2):159–182. doi: 10.1016/0304-4157(73)90003-8. [DOI] [PubMed] [Google Scholar]


