Abstract
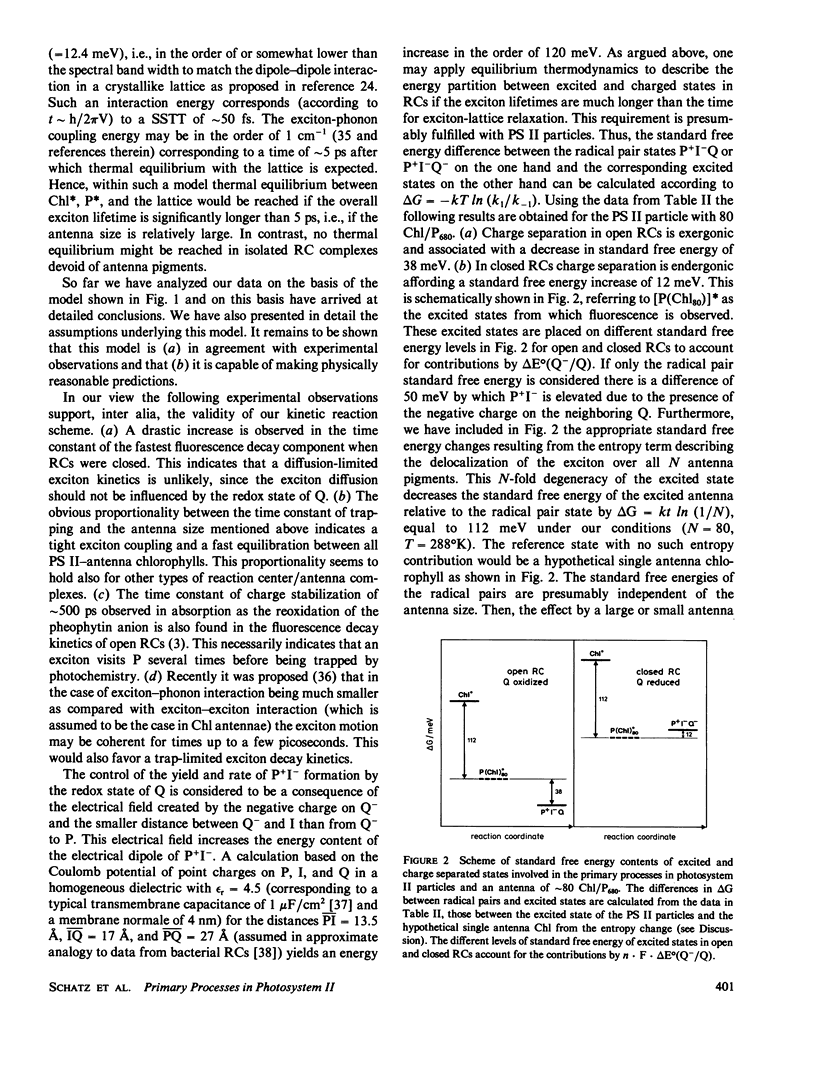
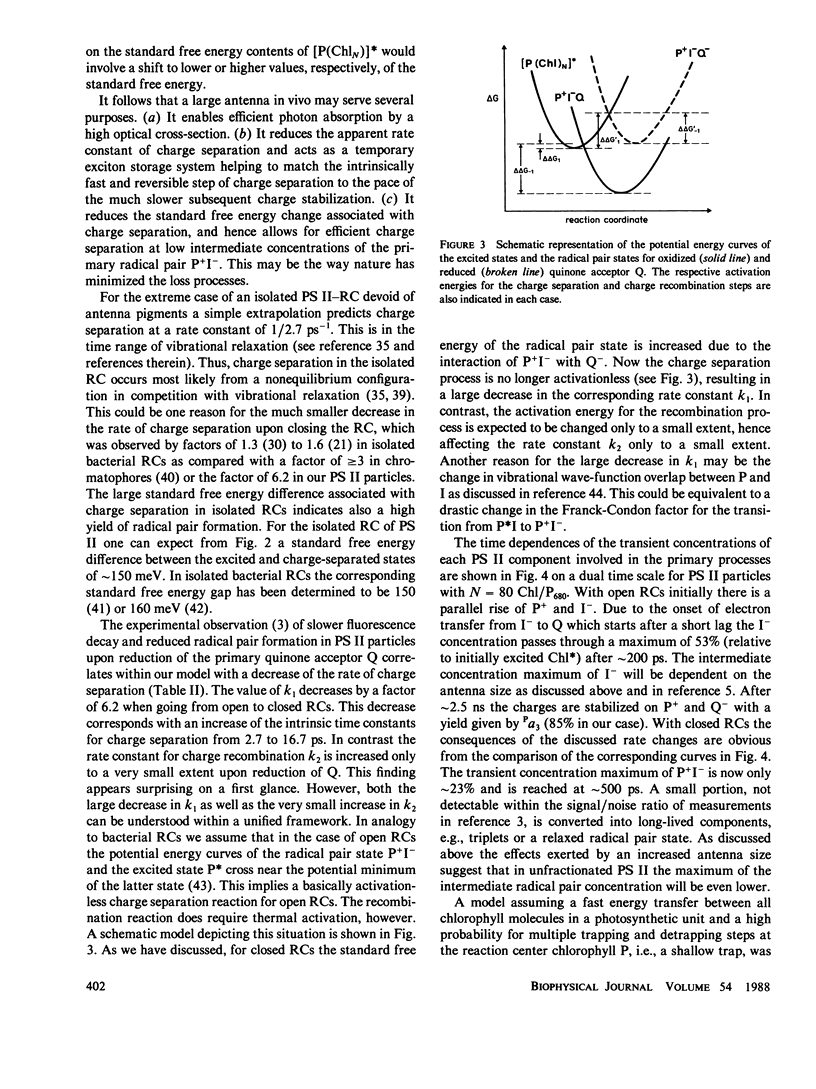
A detailed model for the kinetics and energetics of the exciton trapping, charge separation, charge recombination, and charge stabilization processes in photosystem (PS) II is presented. The rate constants describing these processes in open and closed reaction centers (RC) are calculated on the basis of picosecond data (Schatz, G. H., H. Brock, and A. R. Holzwarth. 1987. Proc. Natl. Acad. Sci. USA. 84:8414-8418) obtained for oxygen-evolving PS II particles from Synechococcus sp. with ∼80 chlorophylls/P680. The analysis gives the following results. (a) The PS II reaction center donor chlorophyll P680 constitutes a shallow trap, and charge separation is overall trap limited. (b) The rate constant of charge separation drops by a factor of ∼6 when going from open (Q-oxidized) to closed (Q-reduced) reaction centers. Thus the redox state of Q controls the yield of radical pair formation and the exciton lifetime in the Chl antenna. (c) The intrinsic rate constant of charge separation in open PS II reaction centers is calculated to be ∼2.7 ps-1. (d) In particles with open RC the charge separation step is exergonic with a decrease in standard free energy of ∼38 meV. (e) In particles with closed RC the radical pair formation is endergonic by ∼12 meV. We conclude on the basis of these results that the long-lived (nanoseconds) fluorescence generally observed with closed PS II reaction centers is prompt fluorescence and that the amount of primary radical pair formation is decreased significantly upon closing of the RC.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breton J., Martin J. L., Migus A., Antonetti A., Orszag A. Femtosecond spectroscopy of excitation energy transfer and initial charge separation in the reaction center of the photosynthetic bacterium Rhodopseudomonas viridis. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5121–5125. doi: 10.1073/pnas.83.14.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Friesner R., Wertheimer R. Model for primary charge separation in reaction centers of photosynthetic bacteria. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2138–2142. doi: 10.1073/pnas.79.6.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Il'ina M. D., Kotova E. A., Borisov AYu The detergent and salt effect on the light-harvesting chlorophyll a/b complex from green plants. Biochim Biophys Acta. 1981 Jul;636(2):193–200. doi: 10.1016/0005-2728(81)90093-1. [DOI] [PubMed] [Google Scholar]
- Kramer H., Mathis P. Quantum yield and rate of formation of the carotenoid triplet state in photosynthetic structures. Biochim Biophys Acta. 1980 Dec 3;593(2):319–329. doi: 10.1016/0005-2728(80)90069-9. [DOI] [PubMed] [Google Scholar]
- Kura-Hotta M., Satoh K., Katoh S. Functional linkage between phycobilisome and reaction center in two phycobilisome oxygen-evolving photosystem II preparations isolated from the thermophilic cyanobacterium Synechococcus sp. Arch Biochem Biophys. 1986 Aug 15;249(1):1–7. doi: 10.1016/0003-9861(86)90553-9. [DOI] [PubMed] [Google Scholar]
- Nordlund T. M., Knox W. H. Lifetime of fluorescence from light-harvesting chlorophyll a/b proteins. Excitation intensity dependence. Biophys J. 1981 Oct;36(1):193–201. doi: 10.1016/S0006-3495(81)84723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T. G., Webb S. P., Mets L., Alberte R. S., Fleming G. R. Antenna size dependence of fluorescence decay in the core antenna of photosystem I: estimates of charge separation and energy transfer rates. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1532–1536. doi: 10.1073/pnas.84.6.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G. W. Excitation transfer and trapping in photosynthesis. Brookhaven Symp Biol. 1966;19:16–48. [PubMed] [Google Scholar]
- Schatz G. H., Brock H., Holzwarth A. R. Picosecond kinetics of fluorescence and absorbance changes in photosystem II particles excited at low photon density. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8414–8418. doi: 10.1073/pnas.84.23.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury N. W., Becker M., Middendorf D., Parson W. W. Picosecond kinetics of the initial photochemical electron-transfer reaction in bacterial photosynthetic reaction centers. Biochemistry. 1985 Dec 17;24(26):7516–7521. doi: 10.1021/bi00347a002. [DOI] [PubMed] [Google Scholar]
- Woodbury N. W., Parson W. W. Nanosecond fluorescence from isolated photosynthetic reaction centers of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1984 Nov 26;767(2):345–361. doi: 10.1016/0005-2728(84)90205-6. [DOI] [PubMed] [Google Scholar]
- van Grondelle R., Holmes N. G., Rademaker H., Duysens L. N. Bacteriochlorophyll fluorescence of purple bacteria at low redox potentials. The relationship between reaction center triplet yield and the emission yield. Biochim Biophys Acta. 1978 Jul 6;503(1):10–25. doi: 10.1016/0005-2728(78)90158-5. [DOI] [PubMed] [Google Scholar]


