Abstract
Apical membrane antigen 1 (AMA1) is regarded as a leading malaria blood-stage vaccine candidate. While the overall structure of AMA1 is conserved in Plasmodium spp., numerous AMA1 allelic variants of P. falciparum have been described. The effect of AMA1 allelic diversity on the ability of a recombinant AMA1 vaccine to protect against human infection by different P. falciparum strains is unknown. We characterize two allelic forms of AMA1 that were both produced in Pichia pastoris at a sufficient economy of scale to be usable for clinical vaccine studies. Both proteins were used to immunize rabbits, singly and in combination, in order to evaluate their immunogenicity and the ability of elicited antibodies to block the growth of different P. falciparum clones. Both antigens, when used alone, elicited high homologous anti-AMA1 titers, with reduced strain cross-reactivity. Similarly, sera from rabbits immunized with a single antigen were capable of blocking the growth of homologous parasite strains at levels theoretically sufficient to clear parasite infections. However, heterologous inhibition was significantly reduced, providing experimental evidence that AMA1 allelic diversity is a result of immune pressure. Encouragingly, rabbits immunized with a combination of both antigens exhibited titers and levels of parasite inhibition as good as those of the single-antigen-immunized rabbits for each of the homologous parasite lines, and consequently exhibited a broadening of allelic diversity coverage.
Deployment of a viable malaria vaccine is regarded as the most cost-effective and practical method of reducing the high human and economic toll of this devastating disease. Raising the immunocompetence of those individuals most at risk for severe disease by vaccination could significantly lower the number of deaths due to clinically severe malaria. Two major requirements for producing a successful malaria vaccine are the ability to cheaply manufacture large amounts of high-quality antigen and a rapid, inexpensive way of analyzing the bioactivity of candidate antigens or combinations of antigens. In this paper we present data addressing both of these issues for Plasmodium falciparum apical membrane antigen 1 (AMA1).
Antigenic polymorphism is an important mechanism by which malaria parasites evade host immune responses (17). Vaccine strategies involving a single target antigen may have their effectiveness limited by antigenic polymorphisms, which allow divergent parasites to circumvent a vaccine's protective properties. Pursuing a strategy involving multiple allelic variants of a single antigen is one way to overcome this mechanism of immune evasion.
Studies using gene substitution suggest that AMA1 is a critical component necessary for successful invasion of red blood cells (RBCs) by merozoites (24). Vaccination with AMA1 has been shown to elicit antibody responses that give good protection against homologous parasite challenges in a number of rodent and primate models (1, 3, 6-8, 14, 27). Additional support for the importance of AMA1-specific antibodies was provided by adoptive-transfer experiments where monoclonal antibodies or purified hyperimmune rabbit immunoglobin protected mice against Plasmodium yoelii or Plasmodium chabaudi challenge (3, 7). However, the protection provided in all these models was strain or species specific. This is probably also true of P. falciparum infections, for while AMA1 is a relatively conserved molecule, 64 single-amino-acid substitutions have been found to date in P. falciparum AMA1 sequenced from field isolates and laboratory strains (12). Analysis of the frequency and distribution of these substitutions has yielded evidence that this genetic diversity is maintained by selective pressures of the host immune response (16). Indirect evidence supporting this hypothesis has come from previous investigations, which have shown little effective cross-strain protection from immunization with a single allelic form of AMA1, even though the immunogen produced high levels of growth inhibition against homologous parasites (11).
Here we have produced two divergent allelic forms of AMA1, one based on the sequence of the Vietnam Oak Knoll (FVO) parasite clone, the other based on the sequence of the 3D7 clone. Using these, we show that the amino acid substitutions in the AMA1 genes of these two clones of P. falciparum, though at a relatively minor level (4%), are not immunologically silent, providing direct evidence to support the hypothesis that such differences are a result of immune pressure. However, we also provide evidence that successful strategies may be developed to enhance cross-strain inhibition.
MATERIALS AND METHODS
Molecular cloning and expression of AMA1 in Pichia pastoris.
Synthetic AMA1 sequences encoding amino acids 25 to 546 were designed on the basis of GenBank sequences for the FVO (AJ277646) and 3D7 (U65407) parasite clones. The native sequences for these two lines were confirmed by direct genomic DNA sequencing of the AMA1 genes of our laboratory strains of FVO and 3D7. The native amino acid sequences were modified as described in Results to remove potential sites of N-linked glycosylation. The synthetic DNA sequences were designed using a computer algorithm that matched each codon used by P. falciparum with a codon used with the same frequency for that amino acid by P. pastoris. The synthetic sequences are available in GenBank under accession numbers AF512507 (FVO) and AF512508 (3D7).
The synthetic AMA1 gene sequences were subcloned into the P. pastoris expression plasmid pPIC9K (Invitrogen Corporation, Carlsbad, Calif.). The pPIC9K plasmid encodes a preprosecretory α-factor sequence. The resulting recombinant proteins, after removal of the signal peptides by the yeast enzyme KEX2, have the sequences YVQNYWEHPYQKSDVYHPIN...TYDNMKTSHHHHHH (FVO) and YVQNYWEHPYQNSDVYRPIN...TYDKMKTSHHHHHH (3D7), where underlined sequences are AMA1 derived and nonunderlined sequences are vector derived. Gene expression is under the control of the alcohol oxidase I (AOXI) gene promoter. The constructs were stably integrated into the genomes of GS115 (his4) host cells, which have a mutation in the histidinol dehydrogenase gene (HIS4) and which are wild type with regard to the AOX1 and AOX2 genes and metabolize methanol at the wild-type rate. The pPIC9K plasmid has a functional HIS4 gene, so transformants are then selected for functional HIS4 activity.
Transformants were further selected for multiple copies of the integration cassette by G418 resistance according to the manufacturer's instructions. Clones with optimal protein expression were selected for large-scale production by fermentation. A series of optimization runs was performed (16 to 20 runs for each of PpAMA1 FVO and 3D7, at the 5-liter scale) using BioFlo3000 fermentors (New Brunswick Scientific, New Brunswick, N.J.). These optimized the fermentation parameters of pH, temperature, growth rates, minimal medium composition, and expression lengths.
Expression was optimized for production at a pilot lot production scale (60 liters) using a New Brunswick BioFlo5000 Mobile Pilot Plant. Six milliliters of frozen cell bank culture was used to inoculate 3 liters of seed medium (0.1 M potassium phosphate [pH 6.0], 1% [vol/vol] glycerol, 1.34% [wt/vol] yeast nitrogen base, 4 × 10−5% [wt/vol] d-biotin). The inoculated seed medium was placed at 30°C and 250 rpm for 22 h, until an optical density at 600 nm of 5 to 15 was reached, and was then used to inoculate 45 liters of fermentation medium (315 mM potassium dihydrogen phosphate, 37.8 mM ammonium sulfate, 5.8 mM dihydrate calcium phosphate, 82.1 mM potassium sulfate, 47.5 mM heptahydrate magnesium sulfate, 4% [wt/vol] glycerol, 1 ml of PTM4 trace salts solution/liter [20]). Fermentation parameters were as follows: pH, 5.0; temperature, 30°C; dissolved oxygen, 30%. The pH was maintained with a 14% ammonium hydroxide feed, and the dissolved-oxygen level was maintained by an airflow of 90 liters min−1, an agitation cascade from 360 to 550 rpm, and airflow supplementation with oxygen gas, in that order. After 20 to 25 h of batch growth (biomass, 80 to 120 g of cells [wet mass]/liter), the cells had consumed all carbon sources and began to starve, which was reflected by a basic shift in pH. This pH shift was used to trigger a fed-batch increase in biomass to 180 to 220 g of cells [wet mass]/liter. The fed-batch phase consisted of a 2-h feed of 50% (vol/vol) glycerol-0.4% (vol/vol) PTM4 trace salts at 870 ml/h, followed by a 3-h ramp to 0 ml/h, when induction was initiated by a feed of 100% methanol-0.4% (vol/vol) PTM4 trace salts at 245 ml/h. To aid the adaptation of the cells to methanol as a carbon source, during the fed-batch phase the culture was also provided with two pulses of methanol. During induction, the pH set point was increased to 6.8 to control proteolysis. Induction under methanol feeding continued for 20 to 30 h. The fermentation culture broth was clarified by microfiltration (with a 0.1-μm-pore size Millipore hollow fiber filter), concentrated by ultrafiltration, and then diafiltered with a 3-kDa hollow fiber filter (A/G Technology Corporation, Needham, Mass.) into 2× phosphate-buffered saline (2× PBS), pH 7.4, comprising 2.08 mM potassium dihydrogen phosphate, 5.94 mM disodium hydrogen phosphate, and 308 mM sodium chloride. The final product was filtered through a 0.22-μm pore-size membrane and frozen at −80°C.
Protein purification.
After thawing, the sterile product was loaded onto a 500-ml nickel-nitrilotriacetic acid Superflow (Qiagen, Valencia, Calif.) column at 90 cm/h. In order to reduce complex protein-protein and protein-lipid interactions which would greatly interfere with later purification resolutions, the product, while bound to the nickel-nitrilotriacetic acid resin, was subjected to a delipidation process, consisting of successive washes with detergent (Tween 80)- and alcohol (isopropanol)-containing buffers, prior to elution with 300 mM imidazole-1× PBS (pH 7.4). The eluant was desalted on a G-25 column (Amersham Pharmacia Biotech) into 0.5× PBS, pH 7.4, prior to being loaded onto an anion-exchange column (Q-Sepharose HP; Amersham). The anion-exchange column was washed, the product was eluted with isocratic NaCl steps, and the eluant product was then directly loaded onto a butyl-Sepharose hydrophobic interaction column (Amersham). AMA1 was eluted from the butyl-Sepharose column with isocratic ammonium sulfate steps. A final purification-buffer exchange step was performed by loading each eluted protein pool onto a 4-liter Superdex 75 size exclusion column (Amersham) preequilibrated with 152 mM NaCl, pH 7.2, at 25 cm/h. Identities of recombinant proteins were confirmed by N-terminal sequencing and electrospray mass spectrometry (Research Technology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Md.).
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on 4-to-20% gradient Tris-glycine polyacrylamide gels by using an X-cell II Mini Cell apparatus (Invitrogen) under nonreducing conditions. Some gels were stained with Coomassie blue. For reducing conditions, samples were mixed with 5% (vol/vol) β-mercaptoethanol (or 50 mM dithiothreitol) before electrophoresis.
Animals and immunization.
All animal studies were carried out in compliance with National Institutes of Health guidelines and with an Animal Care and Use Committee-approved protocol. All studies were carried out with New Zealand White rabbits (Spring Valley Laboratories, Frederick, Md.), with Montanide ISA720 as an adjuvant (SEPPIC Inc., Fairfield, N.J.) and intramuscular injections. Individual study regimens are described in Results.
ELISAs.
Serum antibodies to PpAMA1 were assayed by enzyme-linked immunosorbent assay (ELISA) using an internal standard operating procedure. Briefly, flat-bottom 96-well ELISA plates were coated at 4°C overnight with 100 ng of antigen diluted in 15 mM sodium carbonate-35 mM sodium bicarbonate (pH 9.6)/well. Plates were washed with 0.1% Tween 20 in Tris-buffered saline (TBS) and then blocked with 5% skim milk (Difco, Detroit, Mich.) in TBS for 2 h at room temperature. After the plates were washed again, the test serum was diluted in 0.1% bovine serum albumin-0.05% Tween 20 in TBS, added to antigen-coated wells in triplicate, and incubated for 2 h at room temperature. A duplicate control dilution series of a standard rabbit antiserum to the plate antigen was also added to each plate. After extensive washing, the plates were incubated with alkaline phosphatase-labeled goat anti-rabbit immunoglobulin G (IgG) (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) diluted in the same buffer for 2 h. Bound antibodies were visualized by adding the substrate solution (p-nitrophenyl phosphate; Sigma Chemical Co., St. Louis, Mo.). The absorbance at 405 nm was read with a SpectroMAX 340P ELISA reader (Molecular Dynamics). The number of antibody units in the undiluted test serum was calculated by comparison with the dilution series of the standard rabbit serum; that is, a titer of 1/50,000 antibody units corresponds to a 1/50,000 dilution of the standard rabbit serum, which gives an absorbance of 1.0 at 405 nm.
GIA.
The following P. falciparum culture-adapted lines were used for growth inhibition assays (GIAs): 3D7 (26), FVO (18), HB3 (5), M24 (21), and D10 (2). Prior to use, each line was cultured according to standard methodologies (23), but preadapted to grow in 25% normal rabbit serum without loss of viability.
Sorbitol-synchronized, rabbit serum-adapted cultures of P. falciparum (late trophozoites) were diluted with human RBCs suspended in growth medium (RPMI 1640 containing 0.5% Albumax I [Gibco BRL] and 2% human O+ serum) to give a final concentration of 1% parasitemia, 2% hematocrit. Parasite suspensions (50 μl) were then mixed 1:1 with immunized rabbit serum in wells of 96-well tissue culture plates (Costar, Corning, N.Y.). All test sera were previously heat inactivated (at 56°C for 20 min) and preadsorbed with uninfected human O+ RBCs to remove anti-human RBC immunoglobulins. Serial 1:2 dilutions of immune serum were performed with autologous preimmune serum so that the total serum concentration remained at 25% in all test samples. Controls included autologous preimmune serum (25%), naïve rabbit serum only, and uninfected RBCs. Cultures were grown under 5% O2-5% CO2-90% N2 at 37°C for 40 h. Cultures were then resuspended by pipetting, 50-μl samples were transferred to 300-μl tubes containing 250 μl of cold PBS, and cells were recovered by centrifugation (at 1,500 × g for 10 min). Relative parasitemia levels were determined by using a colorimetric measurement of parasite lactate dehydrogenase (LDH) activity (4). Cells from the remaining 50 μl of test samples were recovered by centrifugation (at 1,500 × g for 10 min) and used to make thin smears for direct parasitemia counts. All assays were run in triplicate.
LDH assay.
Test samples were dissolved in 100 μl of LDH assay buffer (100 mM Tris [pH 7.5] containing 40 mM sodium l-lactate, 0.25% Triton X-100, 0.5 μg of 3-acetylpyridine adenine dinucleotide, 0.2 μg of phenazine ethosulfate, and 20 μg of Nitro Blue Tetrazolium) (all reagents were from Sigma Chemical Co.), and color was allowed to develop in the dark for 40 min at room temperature. Absorbance at 650 nm was then determined using a Spectra Max 340P (Molecular Dynamics) 96-well plate reader. Percent inhibition was calculated as 100 − [(A650 of immune sample − A650 of RBCs only)/(A650 of preimmune control − A650 of RBCs only) × 100] (4).
Host cell-derived protein.
Host-cell-derived protein contaminating the final purified PpAMA1 was assayed by immunological techniques. A Gene-Minus fermentation was performed (with P. pastoris GS115 host cells containing a Gene-Minus plasmid) by following the same fermentation procedure used to produce the PpAMA1's. The supernatant from this fermentation was collected by microfiltration, ultrafiltration, and dialysis as described for the PpAMA1's and was then filtered through a 0.22-μm-pore-size membrane. Additionally, the cells from the fermentation were resuspended to 20% (vol/vol) in 1× PBS, pH 7.4, and then passed three times through an M110Y microfluidizer (Microfluidics, Newton, Mass.), fitted with 100- and 200-μm interaction chambers, at 19,000 lb/in2. Ruptured cells were recovered and centrifuged for 15 min at 4,000 × g, and the supernatant was filtered through a 0.45-μm-pore-size membrane. Five rabbits were immunized with P. pastoris supernatant antigen, and five were immunized with recovered cytosolic antigen. Each rabbit received three immunizations, 3 weeks apart, of 100 μg of total protein emulsified in the adjuvant Montanide ISA720 and was bled 3 weeks after the third vaccination. Serum samples from each of the 10 rabbits were individually assayed against P. pastoris supernatant antigen to determine the host cell antigen detection limit. A dilution series of host cell antigen was spotted directly onto a nitrocellulose membrane, the remaining membrane was blocked with 5% (wt/vol) skim milk powder in 1× PBS, and the membrane was then incubated for 2 h with a 1:1,000 dilution of the rabbit antiserum in the blocking buffer. Membranes were washed with 0.05% (vol/vol) Tween 20 in 1× PBS, and then the rabbit antibody detected with a goat anti-rabbit alkaline phosphatase-conjugated secondary antibody. Detection was performed by using a 5-bromo-4-chloro-3-indolylphosphate (BCIP)/Nitro Blue Tetrazolium colorimetric kit (Kirkegaard & Perry). All five serum samples within a group performed equally well; therefore, for the actual assay, the serum samples were pooled into groups of five (culture supernatant or cytosolic antigen group), each at 1:1,000, to give a final serum concentration of 1:200. These pooled sera were then used to assay a dilution series of bulk-purified PpAMA1 FVO or 3D7 spotted onto a nitrocellulose membrane. A sample of the PpAMA1's was also “spiked” with yeast antigen in order to control for interference in detection.
For each of PpAMA1 FVO and PpAMA1 3D7, two dilution series of 1.5 μg to 0.7 ng of protein were spotted onto a nitrocellulose membrane. One series was probed with rabbit antisera against P. pastoris supernatant antigen, and the second was probed with rabbit antisera against P. pastoris cytosolic antigen. To determine detection limits, a dilution series of P. pastoris supernatant antigen, from 5.7 μg to 0.7 pg of yeast protein, was also spotted onto the nitrocellulose membrane. Additionally, 1.5 μg of drug substance was spiked with 5.7 μg of yeast supernatant antigen and a similar dilution series was set up. Membranes were then probed with sera from five rabbits immunized either with supernatant or with pellets from fermentations of host cells lacking the AMA1 gene. For both the anti-yeast supernatant and the anti-yeast pellet antisera, the detection limit was determined to be 21.7 pg. No inhibition of detection was observed in the spiked drug substance dilution series. A similar amount of yeast protein (i.e., 21.7 pg) was detected in the 5th dilution of the drug substance, or at 23.4 ng of total protein. Thus, the amount of host-cell yeast protein present in the drug substance is 21.7/23,400 · 100%, or 0.09%.
RESULTS
Synthetic gene design.
Variation among the derived amino acid sequences of AMA1 genes sequenced from field isolates is extensive, with some 64 variant positions identified (12). This variation has been examined for geographical or allelic family relationships, with generally negative results (10, 12, 13, 15, 16, 22, 25). However, an analysis performed on the available P. falciparum AMA1 sequences did indicate that the 3D7 and FVO laboratory clones were representative of two extremes of variation for both single point mutations and mutations involving noncontiguous amino acids in linkage disequilibrium.
We thus chose to make recombinant AMA1 proteins in P. pastoris based on the 3D7 and FVO sequences. Because the codon usage of P. pastoris is very different from that of P. falciparum, we synthesized synthetic genes to encode the amino acid sequences of the 3D7 and FVO lines, with the following modifications from the native sequences (Fig. 1).
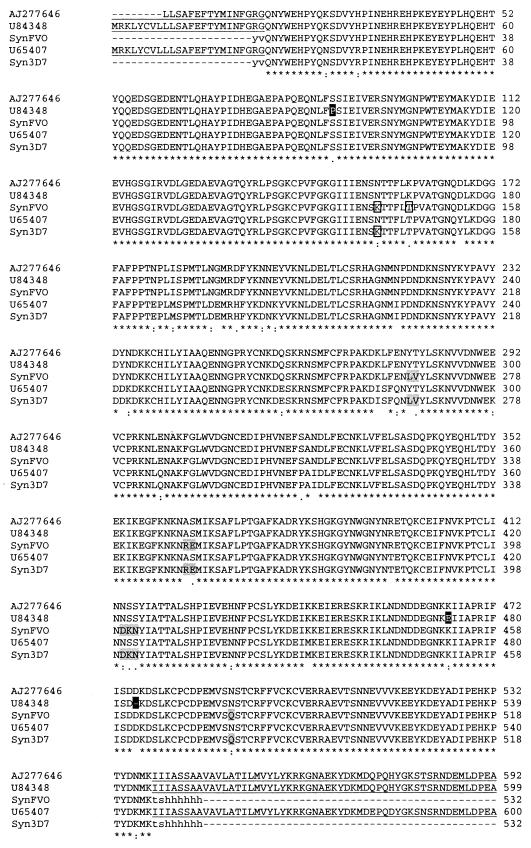
FIG. 1.
Alignment of native and synthetic AMA1 amino acid sequences. The GenBank sequences for P. falciparum FVO AMA1 (AJ277646 and U84348) and P. falciparum F3D7 AMA1 (U65407) are aligned with the sequences used for recombinant protein production in this study (SynFVO and Syn3D7). The three mutations unique to U84348 among all P. falciparum AMA1's, and so not used in the synthetic FVO sequence, are indicated by a solid background. The single glycosylation site mutated on the basis of alignment with allelic variations in other P. falciparum AMA1 sequences is shown as a boxed “K.” Another amino acid in the SynFVO sequence was mutated to T (boxed), because a T always accompanies a K in the previous position (both in other P. falciparum sequences and in the AMA1 sequences of other Plasmodium species). Mutations indicated by shading were chosen by alignment with sequences of other Plasmodium species, because all P. falciparum AMA1 sequences were conserved in this region. The first three of these were from other primate malaria species (Plasmodium vivax, Plasmodium cynomolgi, and/or Plasmodium fragile), and the last was from P. chabaudi. Lowercase letters in the synthetic recombinant sequences represent vector-derived sequences. The signal peptide, transmembrane, and cytoplasmic domains present in the native sequences, but not used in the recombinant proteins, are underlined.
(i) Only the ectodomain of each AMA1 protein was synthesized, up to amino acid 546 (i.e., not the transmembrane domain or the C-terminal cytoplasmic tail).
(ii) The native signal peptide (amino acids 1 to 24) was not included. By use of public signal peptide prediction software (PSORT II and SignalP, version 2.0), two potential cleavage sites were identified (amino acids 18 and 24). Use of the first predicted cleavage site resulted in the production in P. pastoris of a mixed population of proteins, some starting at amino acid 19, some at amino acid 25 (data not shown). We therefore truncated the construct to begin at amino acid 25 and obtained the single, predicted N termini.
(iii) Of the two P. falciparum AMA1 sequences for the FVO parasite line present in GenBank (U84348 and AJ277646), we used AJ277646. U84348 contains three mutations present in no other reported AMA1 genes (a Pro at position 95, a Glu at 471, and a deletion at 484), and attempts to express U84348 in yeast gave no product (data not shown). The DNA sequence of our own FVO laboratory line conformed to that of AJ277646.
(iv) P. pastoris has the ability to N-glycosylate proteins with high-mannose carbohydrates. P. falciparum does not perform N glycosylation, and a loss of efficacy with N glycosylation of a malarial vaccine candidate has been observed previously (19). The native amino acid sequence of the ectodomain of AMA1 has five putative N-linked glycosylation sites. We performed conservative mutations to remove these sites by using the following methodology: if alignment of other P. falciparum AMA1 sequences revealed an alternate amino acid in that position, it was used; if no such alternate was identified, then if alignment of AMA1 sequences of other Plasmodium spp. revealed an alternate amino acid in that position, it was used; if no such alternate was identified, then the asparagine of the recognition site was mutated to a glutamine. Failure to do this resulted in expression of a glycosylated product (e.g., expression of either recombinant 3D7 AMA1 or recombinant FVO AMA1 in P. pastoris where one putative N-linked site was not removed resulted in a glycosylated product [data not shown]).
Recombinant protein production.
Production and purification of both PpAMA1 FVO and PpAMA1 3D7, the recombinant forms of the respective P. falciparum antigens expressed in P. pastoris, have been optimized at the 60-liter level.
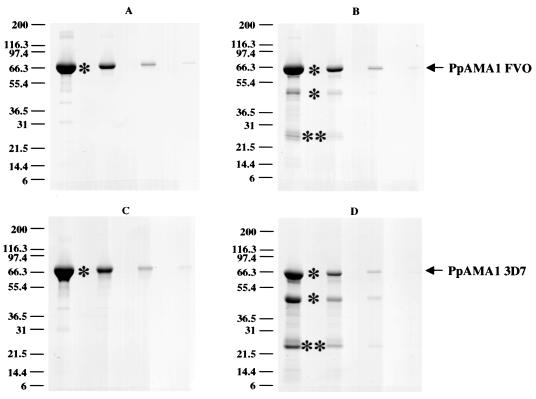
Production levels of final, purified antigen were 3.0 g for PpAMA1 FVO (50 mg/liter) and 1.7 g for PpAMA1 3D7 (28.3 mg/liter). Shown in Fig. 2 are the final antigens. Quality control analysis of these products gave a final purity (percentage in a single band) of 97% and a host cell protein contamination level of 0.09% (Table 1). Endotoxin contamination levels (as measured by a Limulus amebocyte lysate assay) were <0.06 endotoxin unit (EU)/mg.
FIG. 2.
Purity and integrity of PpAMA1's. SDS-PAGE on 4-to-20% gradient Tris-glycine polyacrylamide gels was performed on serial fourfold dilutions of purified PpAMA1 FVO (A and B) and PpAMA1 3D7 (C and D). Quantities loaded were, from left to right, 12.5, 3.1, 0.8, and 0.2 μg. Electrophoresis was performed either under nonreducing conditions (A and C) or with 5% (vol/vol) β-mercaptoethanol (B and D). Scanning laser densitometry showed yields of 96.9% (nonreduced) or 81.9% (reduced) full-length PpAMA1 FVO and 97.6% (nonreduced) or 48.4% (reduced) full-length PpAMA1 3D7. Single asterisks indicate bands whose N-terminal sequence was identified by Edman degradation as Y1VQNYWEHPYQKSDVYHPIN (SynFVO) or Y1VQNYWEHPYQNSDVYRPIN (Syn3D7); double asterisks indicate bands whose N-terminal sequence was S355AFLPTGAFKADRYKSH.
TABLE 1.
Purity and homogeneity of recombinant PpAMA1 FVO and PpAMA1 3D7
| Test | Methoda | Result for:
|
|
|---|---|---|---|
| PpAMA1 FVO | PpAMA1 3D7 | ||
| General safety | 21 CFR 610.11 | Pass | Pass |
| Pyrogenicity | 21 CFR 610.13b | Pass | Pass |
| Sterility | 21 CFR 610.12 | Pass | Pass |
| Endotoxin | LAL gel clot | <0.06 EU/ml | <0.06 EU/ml |
| Host cell protein | Western blotting | 0.09% (wt/wt) | 0.09% (wt/wt) |
| Integrity | SDS-PAGE (nonreducing) | 96.9% full-length AMA1 | 97.6% full-length AMA1 |
| Integrity | SDS-PAGE (reducing) | 81.9% full-length AMA1 | 48.4% full-length AMA1 |
| Mass | Electrospray mass spectrometry | 61,910 Da (predicted mass, 61,904 Da) | —d |
CFR, Code of Federal Regulations; LAL, Limulus amoebocyte lysate.
—, a clean mass spectrometry chromatogram was not obtainable.
No U.S. Food and Drug Administration guidelines for the amount of endotoxin per milligram in a subunit vaccine have been defined, but some commercial vaccines have levels of 1 to 3 EU/dose. Similarly, the amount of contaminating host cell protein acceptable in a subunit vaccine has not yet been defined. However, in consultation with others, we have determined an initial target defined by the following hypothesis: if all contaminating protein were present as a single protein (i.e., worst-case scenario), it would be unlikely that quantities below 1 μg/dose would be sufficient to elicit any immune response (based on the observed immune potential of single-protein vaccines in humans, where dosages of 1 μg or less generally fail to elicit any response). Thus, we have set a limit of less than 100 ng of contaminating host-cell-derived P. pastoris protein/dose in the final drug substance (i.e., 10-fold below the expected range of potential immunogenicity). For both PpAMA1 FVO and PpAMA1 3D7, at doses of <100 μg, we had 90 ng of host cell protein/dose and <0.006 EU.
For both PpAMA1 FVO and PpAMA1 3D, the final purified protein is a mixture of full-length antigen, and a proportion of antigen with a single cleavage in the polypeptide chain, apparent only on reducing SDS-PAGE gels, as the two pieces are normally held together by disulfide bonds (Fig. 2). For PpAMA1 FVO, 81.9% of the protein is full length; for PpAMA1 3D7, 48.4% of the protein is full length. The cleavage point, identified by N-terminal sequencing for both PpAMA1 FVO and PpAMA1 3D7, is part of the large loop in domain 2 (between amino acids 376 and 377 in the 3D7 sequence and between amino acids 354 and 355 in the Syn3D7 sequence [Fig. 1]).
Mass spectrometric analysis of PpAMA1 FVO gave a major peak at 61,910 Da. The predicted mass is 61,904 Da, 6 Da lower, within the error range of the instrument (±0.01%; for a 61,905-Da molecule, ±6.2 Da). We also observed a series of smaller peaks following the major peak, each increasing in mass by ∼162 Da, and this series probably corresponds to glycation of lysine residues. It is unlikely to be N- or O-linked glycosylation, because no N-linked sites are present and there is no evidence of a core polysaccharide structure characteristic of O-linked glycosylation. This has been observed previously with products expressed in P. pastoris (L. Zou, A. P. Miles, J. Wang, and A. W. Stowers, submitted for publication). Two smaller masses were also observed, differing from the major peak by 136 and 276 (2 × 138) Da. Each represents the sequential loss of a single histidine (minus 137.5 Da per histidine) from the C-terminal six-histidine affinity tag. Despite several attempts, we were not able to get a clean mass on PpAMA1 3D7.
Rabbit immunization study 1: biological activity of elicited antibodies.
Two groups of five rabbits each were immunized intramuscularly at 3-week intervals with three 50-μg doses of a single recombinant PpAMA1 formulated in the human-usable water-in-oil adjuvant Montanide ISA720. Thus, animals were immunized on days 0, 21, and 42, and analyses were performed on day 56 serum.
Table 2 shows ELISA titers (in arbitrary antibody units [see Materials and Methods]) for serum samples from the individual rabbits, measured against both PpAMA1 FVO and PpAMA1 3D7. Strikingly, by ELISA, half the antibody response elicited to the immunogen is allele specific. Immunization with PpAMA1 FVO elicited twofold-higher titers to FVO AMA1 than to 3D7 AMA1 (P = 0.007 by Student's t test); immunization with PpAMA1 3D7 elicited twofold-higher titers to 3D7 AMA1 than to FVO AMA1 (P = 0.011 by Student's t test); and the difference between the two groups in the ratio of FVO titers to 3D7 titers is highly significant (P = 9.63 × 10−8 by Student's t test).
TABLE 2.
Antibody titers following immunization with PpAMA1 FVO or PpAMA1 3D7
| Immunogena and rabbit | ELISA titerb to:
|
FVO/3D7 titer ratio | |
|---|---|---|---|
| PpAMA1 FVO | PpAMA1 3D7 | ||
| PpAMA1 FVO | |||
| 85506 | 43,250 | 21,060 | 2.05 |
| 85508 | 53,650 | 24,210 | 2.22 |
| 85511 | 88,550 | 42,850 | 2.07 |
| 85514 | 32,240 | 17,440 | 1.85 |
| 85516 | 36,400 | 15,360 | 2.37 |
| Geometric mean | 47,473.2 | 22,566.7 | 2.10 |
| PpAMA1 3D7 | |||
| 85507 | 6,330 | 16,670 | 0.38 |
| 85509 | 38,400 | 83,650 | 0.46 |
| 85510 | 18,390 | 37,850 | 0.49 |
| 85515 | 42,250 | 78,400 | 0.54 |
| 85517 | 26,770 | 52,650 | 0.51 |
| Geometric mean | 21,915.9 | 46,519.6 | 0.47 |
Rabbits were immunized three times at 3-week intervals, each time intramuscularly with 50 μg of antigen emulsified in Montanide ISA720 (days 0, 21, and 42). Titers shown are from bleeds 2 weeks after the third immunization (day 56).
ELISA titers presented measure total IgG to the indicated recombinant protein in arbitrary antibody units (see Materials and Methods).
The growth-inhibitory characteristics of the resultant antiserum are summarized in Table 3. Both PpAMA1 FVO- and PpAMA1 3D7-immunized animals showed a clear, dose-dependent inhibition of parasite invasion of, or growth in, RBCs. To obtain a fourth point for extrapolation, 50 and 37.5% serum concentrations were also run, where the homologous controls had 50 and 37.5% autologous preimmune serum, respectively (all other assays had a constant total of 25% test plus normal rabbit serum).
TABLE 3.
In vitro growth inhibition activities of sera raised in rabbits against recombinant PpAMA1 FVO and PpAMA1 3D7
| Immunogen and rabbit | % Inhibition of parasite clone:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FVO
|
3D7
|
HB3
|
|||||||||
| % Serum
|
% Serum
|
% Serum
|
|||||||||
| 50 | 37.5 | 25 | 12.5 | 50 | 37.5 | 25 | 12.5 | 50 | 25 | 12.5 | |
| PpAMA1 FVO | |||||||||||
| R85508 | 76.3 | 71.0 | 67.0 | 50.4 | 27.5 | 17.9 | 0.0 | 2.0 | 39.4 | 32.4 | 22.3 |
| R85511 | 76.8 | 76.1 | 73.9 | 62.0 | 51.9 | 44.2 | 33.0 | 18.4 | 72.5 | 61.5 | 54.4 |
| R85506 | 39.1 | 37.6 | 37.9 | 28.6 | 0.0 | 0.0 | 0.0 | 0.0 | 27.2 | 17.3 | 7.3 |
| R85516 | 69.8 | 66.9 | 58.4 | 40.6 | 26.9 | 16.3 | 10.0 | 9.9 | 43.8 | 35.0 | 19.5 |
| R85514 | 67.1 | 62.6 | 49.9 | 33.0 | 35.8 | 27.1 | 16.7 | 12.8 | 52.6 | 41.9 | 24.6 |
| Mean | 65.8 | 62.8 | 57.4 | 42.9 | 28.4 | 21.1 | 11.9 | 8.6 | 47.1 | 37.6 | 25.6 |
| PpAMA1 3D7 | |||||||||||
| R85507 | 0.0 | 0.0 | 0.0 | 0.0 | 51.9 | 38.3 | 22.9 | 4.4 | 6.8 | 0.0 | 0.0 |
| R85509 | 26.5 | 26.5 | 23.9 | 11.5 | 97.7 | 95.5 | 88.7 | 62.1 | 53.2 | 38.4 | 29.6 |
| R85510 | 15.9 | 13.5 | 8.5 | 0.0 | 85.8 | 77.8 | 68.0 | 44.7 | 42.2 | 29.8 | 18.8 |
| R85515 | 69.9 | 67.1 | 56.3 | 40.5 | 96.4 | 91.0 | 82.6 | 51.4 | 62.3 | 45.6 | 24.5 |
| R85517 | 27.6 | 23.4 | 19.2 | 1.6 | 88.0 | 82.3 | 72.0 | 50.0 | 40.8 | 39.1 | 25.6 |
| Mean | 28.0 | 26.1 | 21.6 | 10.7 | 83.9 | 77.0 | 66.9 | 42.5 | 41.1 | 30.6 | 19.7 |
Sera from animals immunized with PpAMA1 FVO showed significantly higher inhibition of growth of homologous parasites than of the heterologous strain 3D7 at all serum dilutions examined (P, 0.001 with 12.5% serum, 0.0008 with 25% serum, 0.0003 with 37.5% serum, and 0.0004 with 50% serum). Similarly, sera from animals immunized with PpAMA1 3D7 showed significantly higher inhibition of growth of homologous parasites than of the heterologous strain FVO at all serum dilutions examined (P, 0.02 with 12.5% serum, 0.003 with 25% serum, 0.002 with 37.5% serum, and 0.001 with 50% serum).
We also chose a third parasite strain (HB3) in order to further examine heterologous inhibition. The sequence of the AMA1 gene from the HB3 parasite line appears to be approximately equally distant from those for the FVO and 3D7 strains, with 21 amino acid substitutions relative to the FVO AMA1 sequence and 25 relative to the 3D7 AMA1 sequence.
At all serum dilutions tested, inhibition of the HB3 parasite by sera from animals immunized with PpAMA1 FVO was both significantly lower than inhibition of the homologous FVO line (P, 0.006 with 12.5% serum, 0.006 with 25% serum, and 0.02 with 50% serum) and significantly higher than inhibition of the 3D7 line (P, 0.02 with 12.5% serum, 0.0002 with 25% serum, and 0.0009 with 50% serum). A similar result was observed with the sera of animals immunized with PpAMA1 3D7: inhibition of the HB3 parasite was both lower than inhibition of the homologous 3D7 line and higher than inhibition of the FVO line, although the results did not reach significance.
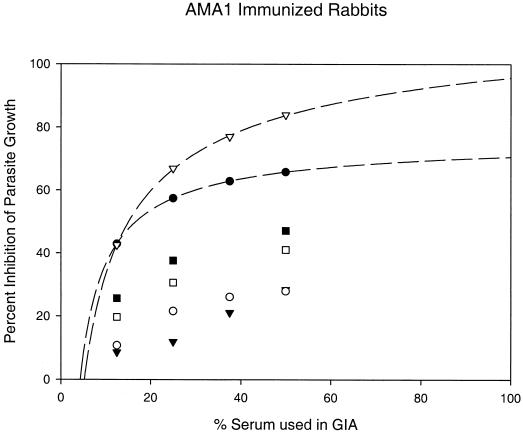
The dose dependence of the inhibition of parasite growth gives an excellent fit to a hyperbolic equation (Fig. 3). This allows an estimation of the level of inhibition obtainable at 100% serum concentrations (i.e., in vivo concentrations), with the clear proviso that the dynamics of invasion inhibition in vitro may be either more (e.g., longer merozoite exposure times) or less (e.g., rosette formation) favorable than those found in vitro. One hundred percent anti-PpAMA1 3D7 serum can thus be estimated to provide ∼95.7% inhibition of homologous parasite growth (r2 = 0.9997). This level of growth inhibition would be sufficient to completely inhibit parasite growth and control an infection (>90% inhibition would result in less than 1 successful merozoite invasion per rupturing schiziont, or negative growth). For PpAMA1 FVO immunization, the estimated level of inhibition by 100% serum is a lower, but still impressive, 70.6% (r2 = 0.9999).
FIG. 3.
Mean growth inhibition levels of rabbit sera raised against recombinant PpAMA1's. Groups of five rabbits each were immunized with 50 μg of either PpAMA1 FVO (solid symbols) or PpAMA1 3D7 (open symbols). Each rabbit serum was assayed individually for growth inhibition against three parasite lines (FVO [circles], 3D7 [inverted triangles], or HB3 [squares]), and the mean percent inhibition of invasion of RBCs for each group of five rabbits is shown along the y axis. GIAs for each serum were conducted at three (with HB3 parasites) or four concentrations, as shown on the x axis. Lines represent regression of homologous results (i.e., PpAMA FVO versus FVO parasites and PpAMA 3D7 versus 3D7 parasites) by use of a hyperbolic equation.
However, for heterologous inhibition, this estimate of in vivo inhibition drops significantly. At a serum concentration of 100%, sera from PpAMA1 3D7-immunized animals would provide 49.14% inhibition of HB3 parasites and 31.57% inhibition of FVO parasites (r2, 1.0 and 0.9996, respectively); sera from PpAMA1 FVO-immunized animals would provide 53.40% inhibition of HB3 parasites and 55.20% inhibition of 3D7 parasites (r2, 1.0 and 0.971, respectively). That is, under optimal conditions (but ignoring the boosting effects of multiple infections), immunization with a single allele of PpAMA1 could be expected to control only a homologous infection, with heterologous infections breaking through. However, we wished to examine the possibility that coimmunization with two candidates would broaden the specificity. With this goal in mind, it was encouraging that in this first study FVO and 3D7 cross-protection was lower than that obtained with the HB3 parasite (i.e., FVO and 3D7 appeared to represent extremes).
Rabbit immunization study 2: overcoming allelic diversity.
Groups of four rabbits were immunized intramuscularly twice, 4 weeks apart, with 25 μg of total antigen emulsified in Montanide ISA720 (i.e., lower and fewer doses than in study 1). The three immunogens (one per group) were 25 μg of PpAMA1 FVO alone, 25 μg of PpAMA1 3D7 alone, and 12.5 μg of FVO plus 12.5 μg of 3D7 given as a single formulated vaccine. Thus, animals were immunized on days 0 and 28, and analyses were performed on day 42 sera.
Interestingly, the differing immunization regimen of lower and fewer antigen vaccinations gave higher antibody titers (compare Tables 2 and 4) and higher levels of growth inhibition (compare Table 3 and Fig. 4 and 5) in this study.
TABLE 4.
Antibody titers following immunization of rabbits with PpAMA1 FVO, PpAMA1 3D7, or a combination of the two antigensa
| Immunogen and rabbit | ELISA titer to
|
FVO/3D7 titer ratio | |
|---|---|---|---|
| PpAMA1 FVO | PpAMA1 3D7 | ||
| PpAMA1 FVO | |||
| STO1 | 107,931 | 42,643 | 2.53 |
| STO2 | 64,139 | 27,739 | 2.31 |
| STO3 | 87,124 | 35,602 | 2.45 |
| STO4 | 167,085 | 59,528 | 2.81 |
| Geometric mean | 100,192.7 | 39,790.9 | 2.52 |
| PpAMA1 3D7 | |||
| STO5 | 38,271 | 98,540 | 0.39 |
| STO6 | 37,534 | 94,441 | 0.40 |
| STO7 | 45,403 | 122,013 | 0.37 |
| STO8 | 29,815 | 65,086 | 0.46 |
| Geometric mean | 37,342.5 | 92,718.6 | 0.40 |
| PpAMA1 FVO plus PpAMA1 3D7 | |||
| STO17 | 73,346 | 78,890 | 0.93 |
| STO18 | 62,356 | 58,240 | 1.07 |
| STO19 | 248,275 | 264,520 | 0.94 |
| STO20 | 65,517 | 80,499 | 0.81 |
| Geometric mean | 92,872.1 | 99,454.2 | 0.93 |
Rabbits were immunized twice, 4 weeks apart (days 0 and 28), each time intramuscularly with 25 μg of antigen emulsified in Montanide ISA720 (either 25 μg of PpAMA1 FVO, 25 μg of PpAMA1 3D7, or 12.5 μg of PpAMA1 FVO plus 12.5 μg of PpAMA1 3D7). Titers shown are from bleeds 2 weeks after the second immunization (day 42).
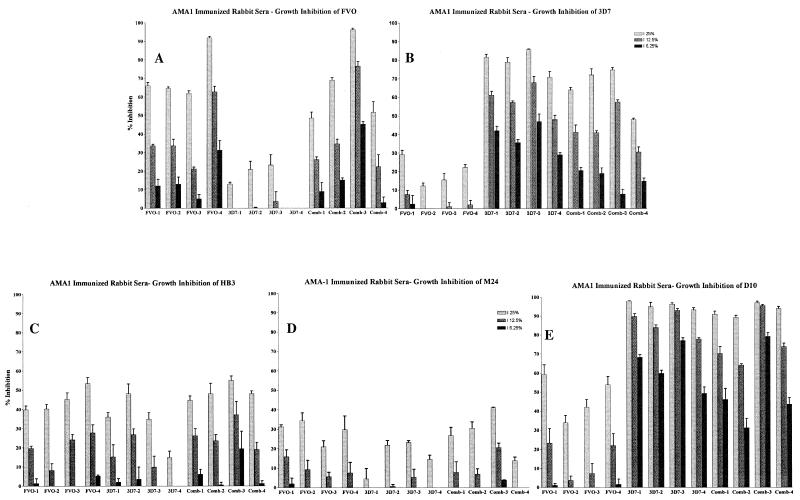
FIG. 4.
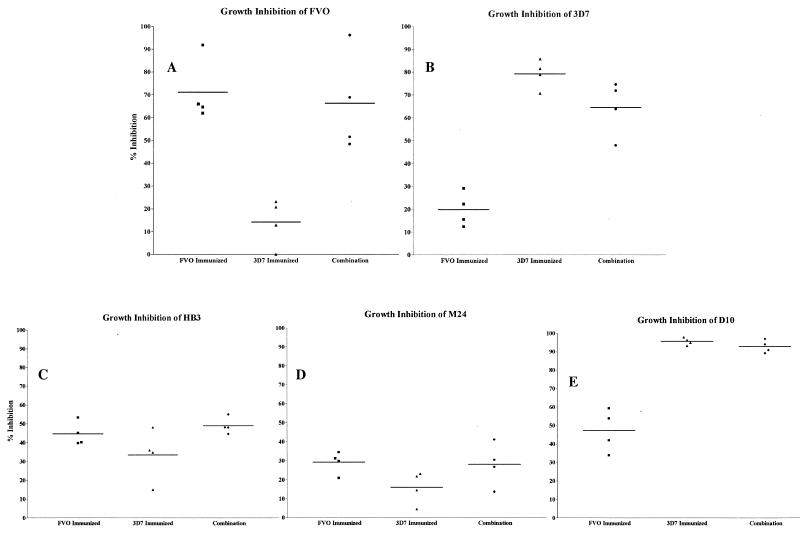
Homologous and heterologous growth inhibition of P. falciparum by sera raised against two alleles of AMA1. Individual sera from each of four rabbits in each of the three immunization groups (PpAMA1 FVO, PpAMA 3D7, or a combination of both) were tested at three different serum concentrations (25, 12.5, and 6.25%) for their abilities to inhibit the growth of five different strains of P. falciparum.
FIG. 5.
Homologous and heterologous GIAs comparing rabbit groups receiving single versus combined immunogens. Rabbits from each of the three immunization groups were compared at the highest serum concentration (25%) for their abilities to inhibit the growth of five different P. falciparum strains. Horizontal lines show the mean value for each group. Groups were compared for statistical significance by an unpaired t test.
As in study 1, the two forms of PpAMA1 performed equally well when used alone to immunize rabbits. No significant difference between the mean titers was observed by ELISA (both in the 100,000 range) when each was tested on the immunizing allele of AMA1 (Table 4). Both single-allele groups displayed significant reductions (>60%; P = 0.024 for FVO; P = 0.007 for 3D7) in mean titers when tested with the other allele as the target antigen. However, the combined-antigen group demonstrated no significant difference between ELISA titers elicited in response to each allele (P = 0.2). Furthermore, the combined-antigen group actually showed geometric mean titers very similar to those of the single-antigen-immunized groups on the homologous immunogen (with no significant differences).
Also, as found previously, for the single-allele-immunized animals, growth inhibition of homologous parasites was dose dependent and was significantly better than growth inhibition of heterologous parasites (P = 0.0006 for PpAMA1 FVO versus PpAMA1 3D7 on FVO parasites; P = 0.0001 for PpAMA1 3D7 versus PpAMA1 FVO on 3D7 parasites) (Fig. 4A and B).
As with the ELISA titers, rabbits immunized with the combination of PpAMA1 FVO and PpAMA1 3D7 showed no significant reduction in growth inhibition from that of either single-allele-immunized group for the homologous parasite strain (P = 0.7234 for FVO versus the combination on FVO parasites; P = 0.0745 for 3D7 versus the combination on 3D7 parasites) (Fig. 4A and B). That is, combining the two alleles into a single formulation did not have the effect of halving the response to either of the alleles. The response made appears to be a “total-AMA1” response.
This response pattern remained constant when growth inhibition of three other parasite strains was examined (Fig. 4C through E) and is shown in a simplified form, with a single serum concentration, in Fig. 5. The three other strains examined were HB3, M24 (27 amino acid differences from 3D7 AMA1 and 19 from FVO AMA1), and D10 (8 amino acid differences from 3D7 AMA1 and 26 from FVO AMA1).
For heterologous inhibition of strains other than FVO or 3D7, the single-antigen immunization groups displayed growth inhibition levels that correlated with the degree of relatedness to the immunizing allele (FVO or 3D7), whereas the combined immunization group consistently did as well as the best of the individual-allele groups. D10 parasites are more closely related to 3D7 (8 amino acid differences) than to FVO (26 amino acid differences), and the levels of inhibition by these two immunization groups were significantly different (P = 0.0002 for PpAMA1 FVO versus PpAMA1 3D7 against D10 parasites) (Fig. 5E). By contrast, the combination group showed no significant loss in the ability to inhibit D10 growth (P = 0.2063 for the combination versus PpAMA1 3D7 on D10 parasites).
In the case of HB3 parasites, sera from PpAMA1 FVO-immunized rabbits did inhibit growth significantly better than sera from rabbits immunized with PpAMA1 3D7 alone (P = 0.0437 for PpAMA1 FVO versus PpAMA1 3D7 on HB3 parasites) (Fig. 5C). Here again the combined-antigen group showed no significant decrease in growth inhibition relative to the better-performing single-antigen group (P = 0.8673 for PpAMA1 FVO versus the combination on HB3 parasites). The AMA1 most distantly related to both FVO and 3D7 was that of strain M24. It is almost equally distant from the FVO and 3D7 sequences, and M24 was the least inhibited of the three heterologous strains tested (Fig. 5D). Given the low levels of inhibition, there was no significant difference between the levels of inhibition with sera from the PpAMA1 FVO- versus the PpAMA1 3D7-immunized group. Again, the combined-antigen group performed similarly to the better of the individual-allele groups (FVO).
DISCUSSION
Due to its target market, successful development of a malaria vaccine requires a large-scale, cost-effective manufacturing process. Testing of candidates prior to meeting these criteria is problematic, because manufacturing changes implemented during the clinical trial program to improve yields and/or costs most likely will lead to product modifications (glycosylation, glycation, and amino acid modifications are just a few of the posttranslational modifications we have observed as a result of manufacturing changes) and consequently to changes in efficacy profiles.
The two AMA1 products we have generated using P. pastoris meet this stringent, preclinical requirement. We have now made clinical lots of both PpAMA1 FVO (50 mg of the final, purified antigen product/liter) and PpAMA1 3D7 (28 mg/liter) and intend to take both into clinical phase I safety studies later this year. The manufacturing process is robust (we have scaled it over 10-fold) and uses entirely synthetic production components throughout cell banking, fermentation, purification of bulk antigen, and final formulation. Both products meet our criteria for purity, host cell protein levels, and endotoxin levels (Table 1). These two antigens will complement the availability of another clinical AMA1 product—a recently published, high-quality recombinant form of P. falciparum AMA1 3D7 expressed in Escherichia coli (9). However, the versatility of the P. pastoris expression system has proven ideal for AMA1 expression, allowing high-level expression of two forms of AMA1, in comparison to the 0.7 mg/liter from E. coli.
A second major goal of this work was to develop a robust, relatively rapid assay for bioactivity of the vaccine components. We utilized the well-published ability of AMA1 to block parasite invasion of RBCs in GIAs as a starting point. Methodologies for performing GIAs vary from laboratory to laboratory, but we wanted to develop a robust (i.e., repeatable) method that avoided the need to purify antibodies from serum (due to cost, possible detrimental effects on antibody activity, time, and volume considerations for an assay to be used in field settings as a surrogate efficacy marker) yet also avoided the confounding effects of false positives that may result from direct use of serum. We found that preadapting malaria cultures to 25% malaria-naïve rabbit serum, using heat-inactivated test serum preadsorbed to human RBCs, using the LDH (4) assay as a rapid, nonradioactive but quantitative detection system, and reading inhibition against homologous preimmune serum produced a robust and repeatable assay. Such an assay system continually gave dose-dependent inhibition of parasite growth (Fig. 3).
Our evaluation of the AMA1 vaccine candidates made here in P. pastoris led to three main conclusions. First, they are very attractive candidates. Used alone, they are able to elicit antibodies capable of significant inhibition in vitro (Table 3 and Fig. 3). Furthermore, extrapolation to serum concentrations of 100% indicates that immunization with PpAMA1 3D7 alone may be able to completely control a homologous infection. This is also demonstrated for PpAMA1 FVO in the accompanying paper describing in vivo protection, at times subpatent, from a lethal P. falciparum challenge in nonhuman primates (19a). This is consistent with other data on AMA1's expressed from other malarial species (1, 3, 6-8, 14, 27) and on a P. falciparum AMA1 expressed in E. coli (11).
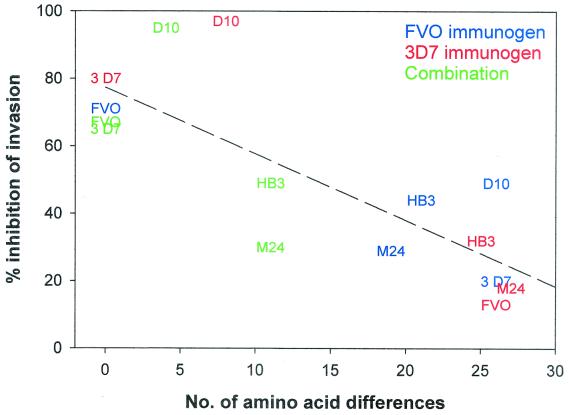
Second, the allelic diversity seen with AMA1's from various parasite isolates is not immunologically silent. This result is not unexpected, from analysis of the frequency and distribution of mutations in the gene sequences (10, 16); from work on protection studies, where immunization or passive transfer of antibodies against one recombinant form of P. chabaudi adami (DS) did not protect against the alternate form of P. chabaudi adami (556KA) (3, 7); and from the purification from human immune sera of antibodies to one P. falciparum allele, which in GIAs inhibited homologous P. falciparum alleles but not all heterologous P. falciparum alleles (11). However, despite these data, most authors still conclude that diversity in AMA1 is limited compared to that in other malaria blood-stage vaccine candidates and consequently is less likely to be a major concern. What we have found surprising is the degree to which the GIA activity of antibodies raised against one allele was adversely affected by the heterogeneity of AMA1. Table 3 and Fig. 3 to 5 demonstrate that GIA activity elicited through 3D7 vaccination can go from 96% (homologous) to 14% (against FVO parasites) inhibition. In fact, the ability of a serum raised against one allele of AMA1 to inhibit in vitro growth by a parasite expressing a heterologous allele of AMA1 appears to correlate almost linearly with the phylogenetic distance between the alleles (number of variant amino acids). Thus, in Fig. 6, the ability to elicit GIA antibodies correlates well with the number of amino acid differences (r2 = 0.633), although the actual number of variant amino acids may in fact be relatively small (on the order of ∼5% in the worst case).
FIG. 6.
Effects of antigenic differences on growth-inhibitory activity. Rabbits were immunized with either PpAMA1 FVO (blue), PpAMA1 3D7 (red), or a combination of both (green). Sera from immunized rabbits were used in GIAs against the M24, HB3, D10, FVO, and 3D7 P. falciparum clones. The number of amino acid differences between an immunizing AMA1 allele (FVO, 3D7, or a combination of both) and the parasite clone tested for inhibition is plotted on the x axis, and the mean level of inhibition obtained against that parasite is plotted on the y axis. For the combined immunization, only those amino acids present in neither immunizing allele are taken into account. The dotted line is the result of a linear regression of all data points.
Of some concern, given these data, is the potential adverse effect of the amino acid substitutions we made in order to avoid N-linked glycosylation in the synthetic genes—9 changes from the 3D7 sequence for PpAMA1 3D7 and 10 changes from the FVO sequence for PpAMA1 FVO, although since many are contiguous, it is perhaps less misleading to say 5 changes each from the FVO and 3D7 sequences (Fig. 1). However, the fact that we retained the biological activity of the elicited antibody response is evidence that the mutations do not have a large detrimental effect (in the case of PpAMA1 3D7, at 50% serum levels, we can obtain a mean inhibition of 96% for 3D7 parasites). This may be because all except one of the N-linked sites occur in areas of conservation in all P. falciparum sequences—i.e., not in areas that have mutated under immune pressure. At the time of gene design, this caused some annoyance in designing alternate codons, because it was difficult to find alternate amino acids for the glycosylation sites that could be predicted to preserve structural integrity due to their presence in other P. falciparum AMA1's, but in retrospect, this may have been fortunate. Additionally, not all amino acid substitutions have adverse outcomes. The AMA1 sequences of 3D7 and D10 have 8 differences in the region of interest (and PpAMA1 3D7 and D10 have 17), but now two studies (the present study and that of Hodder et al. [11]) have found that antibodies raised or purified against the 3D7 allele are just as effective against the D10 allele.
Third, encouragingly for a candidate with such an ability to elicit in vitro GIA activity, the effects of combining variant AMA1 alleles in a single vaccination are positive. The effects of an allelic specificity in an antibody response elicited by a malaria vaccine could be addressed in several ways—by targeting different stages of the parasite's life cycle, by combining entirely different antigens from the same stage of the parasite's life cycle (examined in reference 19a), or by combining multiple allelic forms of one antigen to overcome specificity. In the last case, the wish is either to boost common protective epitopes or simply to cover a sufficient range of the variant epitopes to give a broad specificity. When we combined the two alleles of our AMA1 vaccine, in all cases examined, the combined vaccine (3D7 and FVO) elicited antibodies capable of blocking growth in GIAs to the same extent as the better of the individual components at the same total-AMA1 dosage. Immunizing with 12.5 μg of PpAMA1 FVO plus 12.5 μg of PpAMA1 3D7 was as effective in blocking FVO parasite growth as 25 μg of PpAMA1 FVO, and the same was true of 3D7 growth inhibition. That is, combining the antigens caused no loss of specificity to any one allele; rather, it broadened the number of alleles to which a response was made. Thus, the antibody response elicited by the combined vaccine to D10 was as good as that elicited by 25 μg of PpAMA1 3D7, and the responses to HB3 and M24 were as good as those elicited by 25 μg of PpAMA1 FVO. Most likely, then, rather than boosting common protective epitopes, by immunizing with the combination we are eliciting antibody responses to both sets of allele-specific epitopes. Hopefully, then, a judicious choice of AMA1 alleles could be combined in a vaccine to cover a variety of alleles.
Acknowledgments
We thank Vu Nygen, Richard Shimp, and Michael Whitmore for additional fermentation and purification expertise, and Olga Muratova and Andrew Orcutt for ELISA work.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Amante, F. H., P. E. Crewther, R. F. Anders, and M. F. Good. 1997. A cryptic T cell epitope on the apical membrane antigen 1 of Plasmodium chabaudi adami can prime for an anamnestic antibody response: implications for malaria vaccine design. J. Immunol. 159:5535-5544. [PubMed] [Google Scholar]
- 2.Anders, R. F., G. V. Brown, and A. Edwards. 1983. Characterization of an S antigen synthesized by several isolates of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 80:6652-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anders, R. F., P. E. Crewther, S. Edwards, M. Margetts, M. L. Matthew, B. Pollock, and D. Pye. 1998. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 16:240-247. [DOI] [PubMed] [Google Scholar]
- 4.Basco, L. K., F. Marquet, M. M. Makler, and J. Le Bras. 1995. Plasmodium falciparum and Plasmodium vivax: lactate dehydrogenase activity and its application for in vitro drug susceptibility assay. Exp. Parasitol. 80:260-271. [DOI] [PubMed] [Google Scholar]
- 5.Chen, G. X., C. Mueller, M. Wendlinger, and J. W. Zolg. 1987. Kinetic and molecular properties of the dihydrofolate reductase from pyrimethamine-sensitive and pyrimethamine-resistant clones of the human malaria parasite Plasmodium falciparum. Mol. Pharmacol. 31:430-437. [PubMed] [Google Scholar]
- 6.Collins, W. E., D. Pye, P. E. Crewther, K. L. Vandenberg, G. G. Galland, A. J. Sulzer, D. J. Kemp, S. J. Edwards, R. L. Coppel, J. S. Sullivan, et al. 1994. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am. J. Trop. Med. Hyg. 51:711-719. [DOI] [PubMed] [Google Scholar]
- 7.Crewther, P. E., M. L. Matthew, R. H. Flegg, and R. F. Anders. 1996. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect. Immun. 64:3310-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deans, J. A., A. M. Knight, W. C. Jean, A. P. Waters, S. Cohen, and G. H. Mitchell. 1988. Vaccination trials in rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66 kD merozoite antigen. Parasite Immunol. 10:535-552. [DOI] [PubMed] [Google Scholar]
- 9.Dutta, S., P. V. Lalitha, L. A. Ware, A. Barbosa, J. K. Moch, M. A. Vassell, B. B. Fileta, S. Kitov, N. Kolodny, D. G. Heppner, J. D. Haynes, and D. E. Lanar. 2002. Purification, characterization, and immunogenicity of the refolded ectodomain of the Plasmodium falciparum apical membrane antigen 1 expressed in Escherichia coli. Infect. Immun. 70:3101-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escalante, A. A., H. M. Grebert, S. C. Chaiyaroj, M. Magris, S. Biswas, B. L. Nahlen, and A. A. Lal. 2001. Polymorphism in the gene encoding the apical membrane antigen-1 (AMA-1) of Plasmodium falciparum. X. Asembo Bay cohort project. Mol. Biochem. Parasitol. 113:279-287. [DOI] [PubMed] [Google Scholar]
- 11.Hodder, A. N., P. E. Crewther, and R. F. Anders. 2001. Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. 69:3286-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocken, C. H., D. L. Narum, A. Massougbodji, B. Ayivi, M. A. Dubbeld, A. van der Wel, D. J. Conway, A. Sanni, and A. W. Thomas. 2000. Molecular characterisation of Plasmodium reichenowi apical membrane antigen-1 (AMA-1), comparison with P. falciparum AMA-1, and antibody-mediated inhibition of red cell invasion. Mol. Biochem. Parasitol. 109:147-156. [DOI] [PubMed] [Google Scholar]
- 13.Marshall, V. M., L. Zhang, R. F. Anders, and R. L. Coppel. 1996. Diversity of the vaccine candidate AMA-1 of Plasmodium falciparum. Mol. Biochem. Parasitol. 77:109-113. [DOI] [PubMed] [Google Scholar]
- 14.Narum, D. L., S. A. Ogun, A. W. Thomas, and A. A. Holder. 2000. Immunization with parasite-derived apical membrane antigen 1 or passive immunization with a specific monoclonal antibody protects BALB/c mice against lethal Plasmodium yoelii yoelii YM blood-stage infection. Infect. Immun. 68:2899-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliveira, D. A., V. Udhayakumar, P. Bloland, Y. P. Shi, B. L. Nahlen, A. J. Oloo, W. E. Hawley, and A. A. Lal. 1996. Genetic conservation of the Plasmodium falciparum apical membrane antigen-1 (AMA-1). Mol. Biochem. Parasitol. 76:333-336. [DOI] [PubMed] [Google Scholar]
- 16.Polley, S. D., and D. J. Conway. 2001. Strong diversifying selection on domains of the Plasmodium falciparum apical membrane antigen 1 gene. Genetics 158:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramasamy, R. 1998. Molecular basis for evasion of host immunity and pathogenesis in malaria. Biochim. Biophys. Acta 1406:10-27. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt, L. H. 1972. The course of P. falciparum (Vietnam Oak Knoll strain) in Aotus trivigatus. Trans. R. Soc. Trop. Med. Hyg. 66:521.. [DOI] [PubMed] [Google Scholar]
- 19.Stowers, A. W., L. H. Chen, Y. Zhang, M. C. Kennedy, L. Zou, L. Lambert, T. J. Rice, D. C. Kaslow, A. Saul, C. A. Long, H. Meade, and L. H. Miller. 2002. A recombinant vaccine expressed in the milk of transgenic mice protects Aotus monkeys from a lethal challenge with Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 99:339-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Stowers, A. W., M. C. Kennedy, B. P. Keegan, A. Saul, C. A. Long, and L. H. Miller. 2002. Vaccination of monkeys with recombinant Plasmodium falciparum apical membrane antigen 1 confers protection against blood-stage malaria. Infect. Immun. 70:6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratton, J., V. Chiruvolu, and M. Meagher. 1998. High cell-density fermentation. Methods Mol. Biol. 103:107-120. [DOI] [PubMed] [Google Scholar]
- 21.Su, X., L. A. Kirkman, H. Fujioka, and T. E. Wellems. 1997. Complex polymorphisms in an approximately 330 kDa protein are linked to chloroquine-resistant P. falciparum in Southeast Asia and Africa. Cell 91:593-603. [DOI] [PubMed] [Google Scholar]
- 22.Thomas, A. W., A. P. Waters, and D. Carr. 1990. Analysis of variation in Pf83, an erythrocytic merozoite vaccine candidate antigen of Plasmodium falciparum. Mol. Biochem. Parasitol. 42:285-287. [DOI] [PubMed] [Google Scholar]
- 23.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 24.Triglia, T., J. Healer, S. R. Caruana, A. N. Hodder, R. F. Anders, B. S. Crabb, and A. F. Cowman. 2000. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Mol. Microbiol. 38:706-718. [DOI] [PubMed] [Google Scholar]
- 25.Verra, F., and A. L. Hughes. 2000. Evidence for ancient balanced polymorphism at the apical membrane antigen-1 (AMA-1) locus of Plasmodium falciparum. Mol. Biochem. Parasitol. 105:149-153. [DOI] [PubMed] [Google Scholar]
- 26.Walliker, D., I. A. Quakyi, T. E. Wellems, T. F. McCutchan, A. Szarfman, W. T. London, L. M. Corcoran, T. R. Burkot, and R. Carter. 1987. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science 236:1661-1666. [DOI] [PubMed] [Google Scholar]
- 27.Xu, H., A. N. Hodder, H. Yan, P. E. Crewther, R. F. Anders, and M. F. Good. 2000. CD4+ T cells acting independently of antibody contribute to protective immunity to Plasmodium chabaudi infection after apical membrane antigen 1 immunization. J. Immunol. 165:389-396. [DOI] [PubMed] [Google Scholar]