Abstract
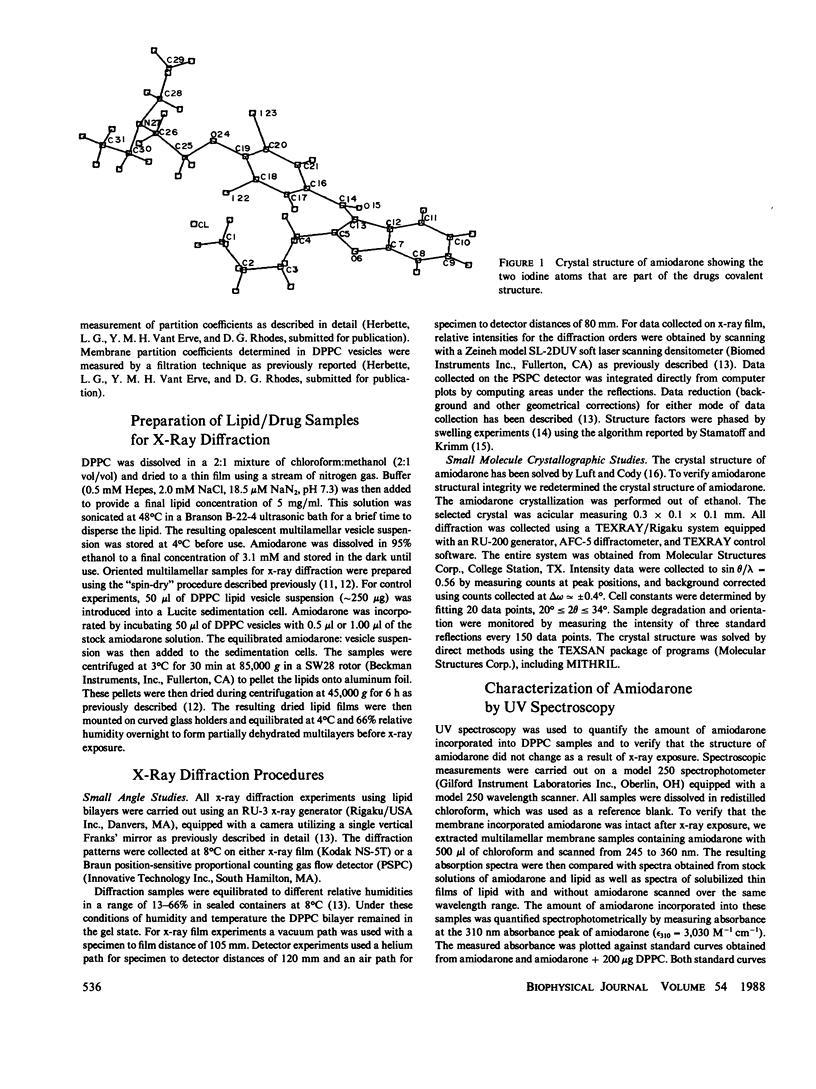
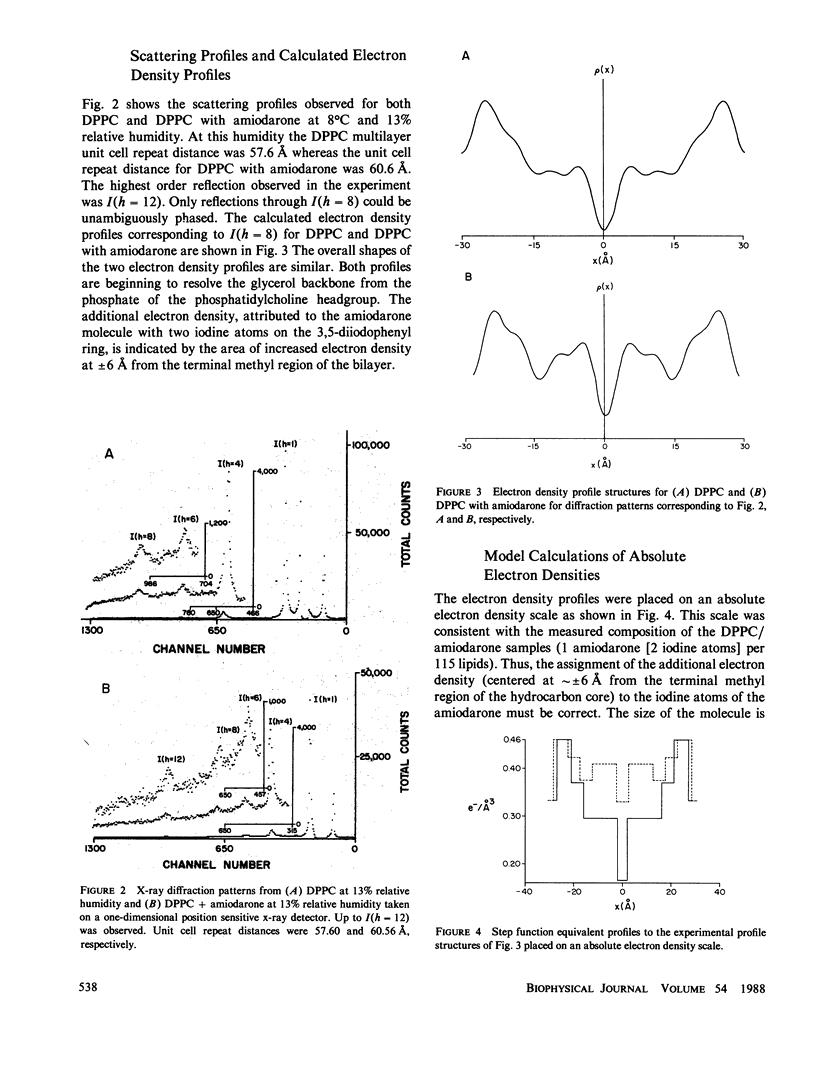
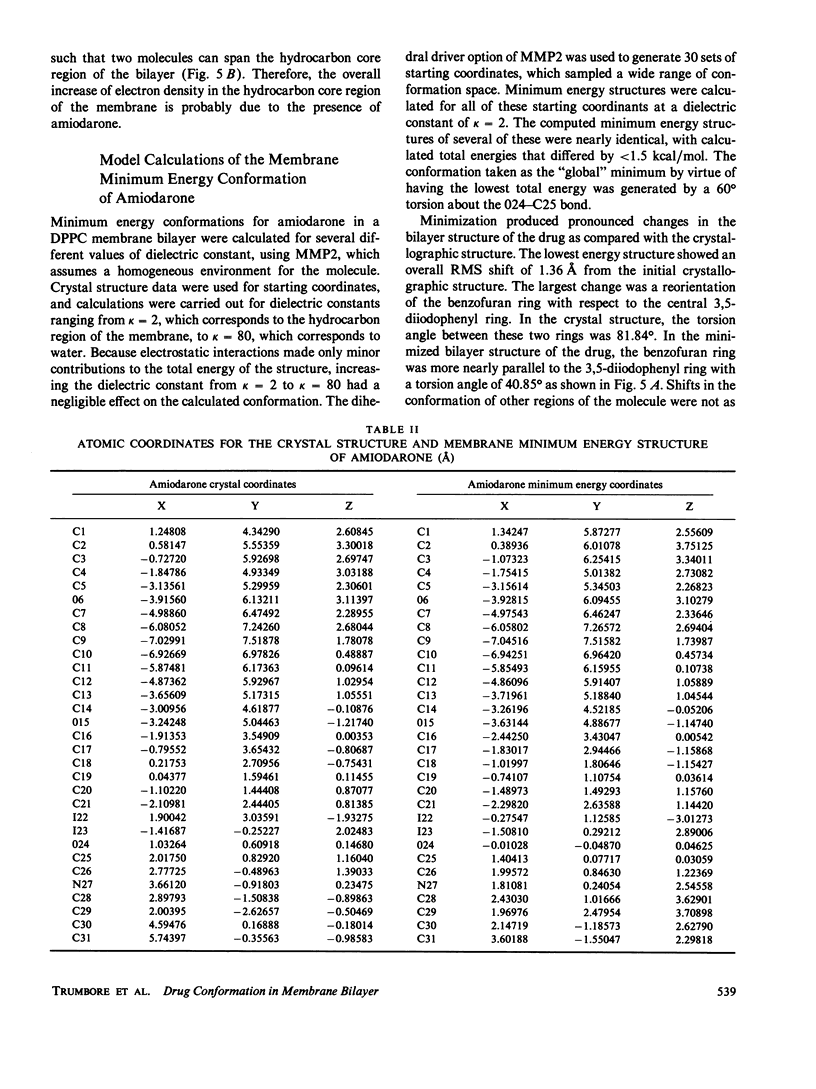
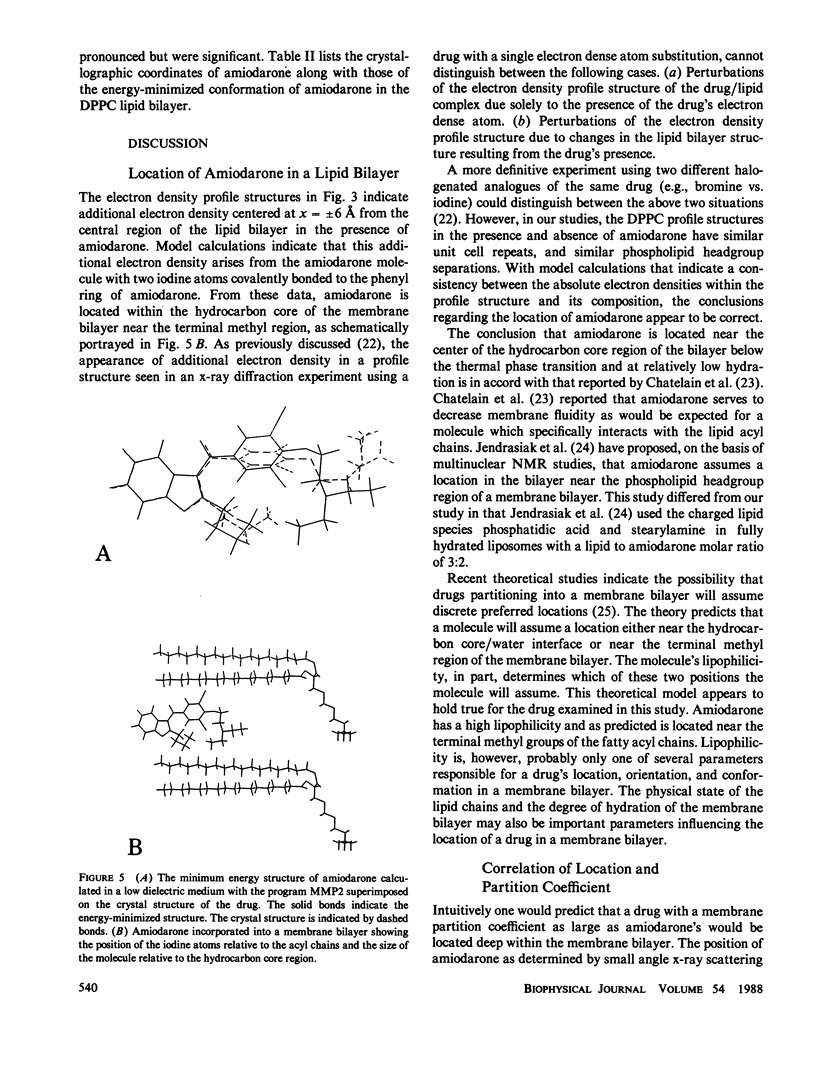
Amiodarone is a drug used in the treatment of cardiac arrhythmias and is believed to have a persistent interaction with cellular membranes. This study sought to examine the structure and location of amiodarone in a membrane bilayer. Amiodarone has a high membrane partition coefficient on the order of 10(6). Small angle x-ray diffraction was used to determine the position of the iodine atoms of amiodarone in dipalmitoylphosphatidylcholine (DPPC) lipid bilayers under conditions of low temperature and hydration where the DPPC bilayer is in the gel state. The time-averaged position of the iodine atoms was determined to be approximately 6 A from the center (terminal methyl region) of the lipid bilayer. A dielectric constant of kappa = 2, which approximates that of the bilayer hydrocarbon core region, was used in calculating a minimum energy structure for membrane-bound amiodarone. This calculated structure when compared with the crystal structure of amiodarone demonstrated that amiodarone could assume a conformation in the bilayer significantly different from that in the crystal. The results reported here are an attempt to correlate the position of a membrane-active drug in a lipid bilayer with its time-averaged conformation. This type of analysis promises to be of great use in the design of drugs with greater potency and higher specificity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chatelain P., Laruel R. Amiodarone partitioning with phospholipid bilayers and erythrocyte membranes. J Pharm Sci. 1985 Jul;74(7):783–784. doi: 10.1002/jps.2600740720. [DOI] [PubMed] [Google Scholar]
- Chatelain P., Laruel R., Gillard M. Effect of amiodarone on membrane fluidity and Na+/K+ ATPase activity in rat-brain synaptic membranes. Biochem Biophys Res Commun. 1985 May 31;129(1):148–154. doi: 10.1016/0006-291x(85)91415-9. [DOI] [PubMed] [Google Scholar]
- Chester D. W., Herbette L. G., Mason R. P., Joslyn A. F., Triggle D. J., Koppel D. E. Diffusion of dihydropyridine calcium channel antagonists in cardiac sarcolemmal lipid multibilayers. Biophys J. 1987 Dec;52(6):1021–1030. doi: 10.1016/S0006-3495(87)83295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark N. A., Rothschild K. J., Luippold D. A., Simon B. A. Surface-induced lamellar orientation of multilayer membrane arrays. Theoretical analysis and a new method with application to purple membrane fragments. Biophys J. 1980 Jul;31(1):65–96. doi: 10.1016/S0006-3495(80)85041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J., Brasseur R., Chatelain P., Ruysschaert J. M. Properties of amiodarone monolayer spread at the air-water interface. J Pharm Pharmacol. 1986 Aug;38(8):561–566. doi: 10.1111/j.2042-7158.1986.tb03081.x. [DOI] [PubMed] [Google Scholar]
- Harigaya S., Schwartz A. Rate of calcium binding and uptake in normal animal and failing human cardiac muscle. Membrane vesicles (relaxing system) and mitochondria. Circ Res. 1969 Dec;25(6):781–794. doi: 10.1161/01.res.25.6.781. [DOI] [PubMed] [Google Scholar]
- Herbette L. G., Chester D. W., Rhodes D. G. Structural analysis of drug molecules in biological membranes. Biophys J. 1986 Jan;49(1):91–94. doi: 10.1016/S0006-3495(86)83605-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbette L. G., MacAlister T., Ashavaid T. F., Colvin R. A. Structure-function studies of canine cardiac sarcolemmal membranes. II. Structural organization of the sarcolemmal membrane as determined by electron microscopy and lamellar X-ray diffraction. Biochim Biophys Acta. 1985 Feb 14;812(3):609–623. doi: 10.1016/0005-2736(85)90254-8. [DOI] [PubMed] [Google Scholar]
- Herbette L., Scarpa A., Blasie J. K., Wang C. T., Hymel L., Seelig J., Fleischer S. The determination of the separate Ca2+ pump protein and phospholipid profile structures within reconstituted sarcoplasmic reticulum membranes via X-ray and neutron diffraction. Biochim Biophys Acta. 1983 May 5;730(2):369–378. doi: 10.1016/0005-2736(83)90354-1. [DOI] [PubMed] [Google Scholar]
- Johansson A. M., Karlén A., Grol C. J., Sundell S., Kenne L., Hacksell U. Dopaminergic 2-aminotetralins: affinities for dopamine D2-receptors, molecular structures, and conformational preferences. Mol Pharmacol. 1986 Sep;30(3):258–269. [PubMed] [Google Scholar]
- Liljefors T., Wikström H. A molecular mechanics approach to the understanding of presynaptic selectivity for centrally acting dopamine receptor agonists of the phenylpiperidine series. J Med Chem. 1986 Oct;29(10):1896–1904. doi: 10.1021/jm00160a017. [DOI] [PubMed] [Google Scholar]
- MOODY M. F. X-RAY DIFFRACTION PATTERN OF NERVE MYELIN: A METHOD FOR DETERMINING THE PHASES. Science. 1963 Nov 29;142(3596):1173–1174. doi: 10.1126/science.142.3596.1173. [DOI] [PubMed] [Google Scholar]
- Mason J. W., Hondeghem L. M., Katzung B. G. Amiodarone blocks inactivated cardiac sodium channels. Pflugers Arch. 1983 Jan;396(1):79–81. doi: 10.1007/BF00584702. [DOI] [PubMed] [Google Scholar]
- Pachence J. M., Dutton P. L., Blasie J. K. Structural studies on reconstituted reaction center-phosphatidylcholine membranes. Biochim Biophys Acta. 1979 Nov 8;548(2):348–373. doi: 10.1016/0005-2728(79)90141-5. [DOI] [PubMed] [Google Scholar]
- Polster P., Broekhuysen J. The adrenergic antagonism of amiodarone. Biochem Pharmacol. 1976 Jan 15;25(2):131–134. doi: 10.1016/0006-2952(76)90279-3. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M. B., Chiale P. A., Halpern M. S., Nau G. J., Przybylski J., Levi R. J., Lázzari J. O., Elizari M. V. Clinical efficacy of amiodarone as an antiarrhythmic agent. Am J Cardiol. 1976 Dec;38(7):934–944. doi: 10.1016/0002-9149(76)90807-9. [DOI] [PubMed] [Google Scholar]
- Stamatoff J. B., Krimm S. Phase determination of x-ray reflections for membrane-type systems with constant fluid density. Biophys J. 1976 May;16(5):503–516. doi: 10.1016/S0006-3495(76)85705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watras J., Messineo F. C., Herbette L. G. Mechanisms of fatty acid effects on sarcoplasmic reticulum. I. Calcium-fatty acid interaction. J Biol Chem. 1984 Jan 25;259(2):1319–1324. [PubMed] [Google Scholar]



