Abstract
A major challenge facing malaria vaccine development programs is identifying efficacious combinations of antigens. To date, merozoite surface protein 1 (MSP1) is regarded as the leading asexual vaccine candidate. Apical membrane antigen 1 (AMA1) has been identified as another leading candidate for an asexual malaria vaccine, but without any direct in vivo evidence that a recombinant form of Plasmodium falciparum AMA1 would have efficacy. We evaluated the efficacy of a form of P. falciparum AMA1, produced in Pichia pastoris, by vaccinating Aotus vociferans monkeys and then challenging them with P. falciparum parasites. Significant protection from this otherwise lethal challenge with P. falciparum was observed. Five of six animals had delayed patency; two of these remained subpatent for the course of the infection, and two controlled parasite growth at <0.75% of red blood cells parasitized. The protection induced by AMA1 was superior to that obtained with a form of MSP1 used in the same trial. The protection induced by a combination vaccine of AMA1 and MSP1 was not superior to the protection obtained with AMA1 alone, although the immunity generated appeared to operate against both vaccine components.
Many malaria vaccine strategies, including our own, depend on including multiple asexual antigens in order (i) to improve coverage of polymorphisms in field isolates, (ii) to overcome individual nonresponsiveness to some antigens, (iii) to improve vaccine efficacy by eliciting immunity to multiple targets, and (iv) to prevent or delay the evolution of escape mutants. If we include two different antigens in a vaccine combination, ideally, synergy in protection will be induced.
The most extensive experience in vaccine trials with New World monkeys has been obtained with the C-terminal 42-kDa portion of merozoite surface protein 1 (MSP142) (3, 10, 17, 18). Recombinant forms of MSP142 have been efficacious against homologous parasite challenges, and MSP142 is regarded as a leading asexual vaccine candidate.
We have now produced a second antigen, Plasmodium falciparum apical membrane antigen 1 (AMA1) (see reference 13a), and in the present study we test the efficacy of this antigen, both alone and in combination with MSP142, in a vaccine trial with Aotus vociferans monkeys.
AMA1 is the subject of intensive vaccine research; at least six of the major malaria vaccine research centers have AMA1 programs. This is based on protection against rodent malaria (Plasmodium chabaudi [1, 2, 6, 20] and Plasmodium yoelii [16]) and nonhuman primate malarias (Plasmodium knowlesi [7] and Plasmodium fragile [5]) by use of purified parasite and recombinant antigens and on the generation in rabbits of an in vitro growth-inhibitory antiserum to an Escherichia coli-expressed recombinant P. falciparum AMA1 (12).
However, no evidence exists that vaccine-elicited immunity to a recombinant form of P. falciparum AMA1 will be effective against a P. falciparum challenge in vivo, and establishing this prior to human trials is seen by us as essential, especially because the accumulated data of vaccination trials with both purified native and recombinant AMA-1 have established that the protective efficacy seen to date for this molecule requires the native conformation of AMA1. That is, it is already well established that the quality of the antigen will determine the vaccination outcome. Studies comparing reduced versus correctly folded recombinant P. chabaudi AMA1 (2, 6) and affinity-purified P. knowlesi AMA1 (7) demonstrated protection from parasite challenge only with the correctly folded protein, despite similar anti-AMA1 antibody levels in the two groups. Furthermore, passive transfer of polyclonal anti-AMA1 antibodies raised in rabbits immunized with recombinant P. chabaudi AMA1 protected mice against homologous parasite challenge only when antibodies were raised against correctly folded AMA1 (2, 6).
This study is thus a preclinical in vivo evaluation of the quality of the recombinant P. falciparum AMA1 we have produced and a preclinical assessment of the effects on vaccine efficacy of combining AMA1 with MSP142.
MATERIALS AND METHODS
Recombinant protein production.
The production and purification of a recombinant form of the 66-kDa ectodomain of P. falciparum AMA1 by use of the Pichia pastoris expression system are described in the companion paper (13a). The expression level of the recombinant AMA1 based on the sequence of the FVO parasite line was 50 mg of the final, purified product per liter of fermentation broth. The final purity (percentage in a single band) was 97%, with a host cell protein contamination level of 0.09% and endotoxin contamination levels (as measured by a Limulus amoebocyte lysate assay) of <0.06 endotoxin unit (EU)/mg. This product was shown to be immunogenic in rabbits, producing antibodies effective at blocking ∼70% of homologous merozoite invasion of red blood cells (RBCs) in vitro.
The production and protective efficacy in Aotus nancymai monkeys of a recombinant form of MSP142 expressed in a baculovirus system, bvMSP142, have been described previously (18).
Vaccination and challenge infection of malaria-naïve A. vociferans monkeys.
Monkeys were housed at the Primate Research Facility, National Institutes of Health (NIH), in compliance with an NIH Animal Care and Use Committee-approved protocol (LPD-8E). Thirty-two monkeys were randomly assigned to four groups of seven and one group of four. Group assignment was masked to the primary investigators who cared for or vaccinated the animals, read films, or determined when a monkey should be drug cured. Randomization was done in such a way as to ensure that a control monkey was challenged first and last. Groups were as follows: a group of four negative-control animals, who received no vaccinations or treatment prior to challenge with P. falciparum; a second negative-control group consisting of seven animals, to control for the effects of protein plus adjuvant, who received a recombinant form of the Plasmodium vivax sexual-stage protein Pvs25H (11); a positive-control group of seven animals receiving bvMSP142 (which had previously protected A. nancymai monkeys); and two test groups of seven animals each, receiving AMA1 alone and AMA1 plus bvMSP142, respectively.
Except for the first group of four animals, each group received three immunizations with 100 μg of antigen, emulsified in complete (first vaccination) Freund's adjuvant (Sigma Chemical Co., St. Louis, Mo.) or incomplete (subsequent vaccinations) Freund's adjuvant (Montanide ISA51; SEPPIC Inc., Fairfield, N.J.), at 3-week intervals before challenge with 5 × 104 FVO parasites 17 days after the third vaccination according to our established protocol (18). Additionally, the four unvaccinated animals were also challenged with parasites in order to examine any effects of the adjuvant alone. The combined AMA1-plus-bvMSP142 group received 100 μg of each antigen, for a total of 200 μg, delivered in a single formulation.
Animals were challenged by intravenous infusion of a freshly passaged preparation of 5 × 104 RBCs infected with the highly virulent P. falciparum strain FVO. Hematocrit and Giemsa-stained thin films were made from blood collected by puncture of superficial veins in the dorsum of the calf. Parasitemia was monitored daily by inspection of Giemsa-stained thin films until treatment and was calculated based on examination of approximately 2,000 RBCs; if no parasites were seen, then 40 more high-power fields were examined. Monkeys were treated when parasitemia reached 5% or when their hematocrit fell below 25%. All but two monkeys not treated previously were treated on day 28. Treatment consisted of mefloquine administered in a single dose of 25 mg/kg of body weight by intubation. The remaining two monkeys were treated on days 33 and 36, respectively.
Statistical methods.
Aotus monkeys that control their parasitemia either self-cure or suffer anemia, requiring treatment. At this point it is impossible to say what would have occurred to an anemic monkey's parasite burden—the monkey might have self-cured, or continued to control parasitemia, or lost control and suffered an acute infection. Thus, the primary end point includes only data until the first monkey was treated for hematocrit rather than parasitemia. On that day, all monkeys were ranked in the following order. Monkeys that were treated for parasitemia prior to the day of data collection ranked first, in the order of (first) treatment and then cumulative parasitemia (the sum of a monkey's daily parasite burdens). Then the monkeys that required treatment for hematocrit (so triggering the end point) were ranked in the same fashion. Finally, monkeys not requiring treatment up to that point were ranked in the order of their cumulative parasitemias (18). A nonparametric, unpaired Mann-Whitney U test was then performed to compare test groups to the control group.
Secondary statistical comparisons were also made. Nonlinear Spearman's regression analysis was performed to correlate antibody responses to protection from challenge. Unpaired Mann-Whitney U tests were also used to compare the antibody responses elicited to the vaccines and to compare the days until patency.
ELISAs.
Serum antibodies to AMA1 and MSP142 were assayed by enzyme-linked immunosorbent assay (ELISA) using an internal standard operating procedure. Briefly, flat-bottom 96-well ELISA plates were coated at 4°C overnight with 100 ng of antigen diluted in 15 mM sodium carbonate-35 mM sodium bicarbonate (pH 9.6)/well. Plates were washed with 0.1% Tween 20 in Tris-buffered saline (TBS) and then blocked with 5% skim milk (Difco, Detroit, Mich.) in TBS for 2 h at room temperature. After the plates were again washed, the test serum was diluted in 0.1% bovine serum albumin-0.05% Tween 20 in TBS, added to antigen-coated wells in triplicate, and incubated for 2 h at room temperature. A duplicate control dilution series of a standard Aotus antiserum to the plate antigen was also added to each plate. After extensive washing, plates were incubated with alkaline phosphatase-labeled goat anti-monkey immunoglobulin G (IgG) (Rockland) diluted in the same buffer for 2 h. Bound antibodies were visualized by addition of the substrate solution (p-nitrophenyl phosphate; Sigma Chemical Co.). Absorbance at 405 nm was read with a SpectroMAX 340P ELISA reader (Molecular Dynamics). The antibody units in the undiluted test serum were calculated by comparison with the dilution series of the standard Aotus serum. The two standard sera used had antibody units of 50,000 (MSP142) and 200,000 (AMA1); that is, a 1/50,000 or 1/200,000 dilution of the standard serum gives an absorbance of 1.0 at 405 nm.
Sequence analysis of escape mutants.
Infected blood from monkeys was collected in heparinized containers, and genomic DNA was extracted (4). Direct PCR was performed on the extracted DNA by using a series of five overlapping primer pairs based on conserved regions of the ama1 gene. By using standard methods, the PCR products were purified and then sequenced.
RESULTS
Study design modifications.
During the immunization period, three animals died of cardiomyopathy (two from the Pvs25H group [3 weeks after the first vaccination and 3 to 22 h following the second vaccination, respectively] and one from the AMA1 group [10 days after the second vaccination]). This was a reflection of the limited availability of these wild-caught monkeys (thus, tight screening of health status was not possible prior to trial commencement). However, prior to parasite challenge, the health of all the enrolled Aotus monkeys was evaluated. Monkeys with anemia (hematocrit levels of <30%) were then withdrawn from the study. This involved three animals from the bvMSP142 group. Thus, final group sizes were as follows: five animals in the protein-plus-adjuvant negative-control (Pvs25H) group, four animals in the bvMSP42 group, six animals in the AMA1 group, seven animals in the AMA1-plus-bvMSP142 group, and four animals in the unvaccinated control group.
Protection from P. falciparum induced by vaccination with antigen plus Freund's adjuvant.
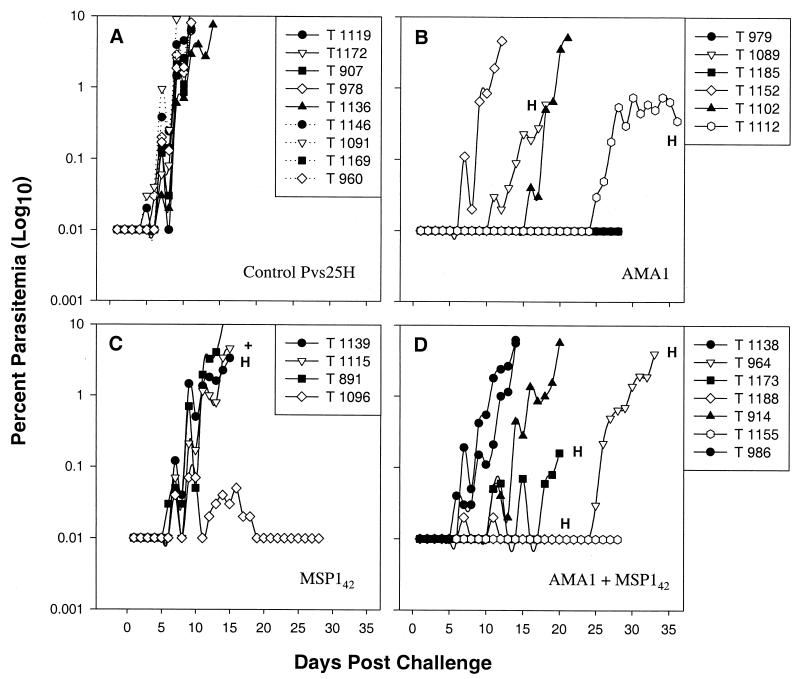
Vaccination with Pvs25H had no protective efficacy (Fig. 1A). All five animals required treatment for uncontrolled parasitemia, and no significant differences were observed in terms of days to treatment or parasitemia at treatment between this group and the four unvaccinated control monkeys (Fig. 1A and Table 1) (P = 0.5).
FIG. 1.
Daily parasitemias in A. vociferans monkeys after challenge. Monkeys were vaccinated three times with 100 μg of Pvs25H (solid lines) or nothing (dotted lines) (A), 100 μg of AMA1 (B), 100 μg of MSP142 (C), or 100 μg of AMA1 plus 100 μg of bvMSP142 (D). Two weeks after the final vaccination, they were challenged with 5 × 104 P. falciparum FVO parasites, freshly passaged from a donor monkey. Thin blood films were taken daily and stained with Giemsa stain. Parasitemia was determined as the percentage of infected RBCs in 50 (for <0.1% parasitemia) or 10 (for >0.1% parasitemia) high-power magnification fields. Monkeys were treated either at day 28, at >5.0% parasitemia, or when their hematocrit (H) fell below 25%. +, monkey T1115 died of heart problems at a parasitemia of 4.6%. Due to the late appearance of parasites, monkeys T1112 and T964 were monitored until days 36 and 33, respectively.
TABLE 1.
Course of infection in A. vociferans monkeys challenged with P. falciparum parasites
| Vaccine group and monkey | ELISA titera to:
|
Days to:
|
% Parasitemia
|
Outcomed | |||
|---|---|---|---|---|---|---|---|
| AMA1 | MSP142 | Patencyb | Treatmentc | Peak | At treatment | ||
| Pvs25H | |||||||
| T1119 | 206 | 982 | 5 | 11 | 6.20 | 6.20 | Virulent |
| T1172 | 63 | 4,160 | 5 | 11 | 6.25 | 6.25 | Virulent |
| T907 | 7,450 | 22,750 | 6 | 11 | 6.50 | 6.50 | Virulent |
| T978 | 75 | 12,280 | 6 | 11 | 20.15 | 20.15 | Virulent |
| T1136 | 11,020 | 42,800 | 7 | 14 | 7.55 | 7.55 | Virulent |
| Meane | 603 | 8,665 | 6 | 12 (Δ = 7) | 9 | 9 | |
| Naïve | |||||||
| T1146 | — | — | 4 | 11 | 25.05 | 25.05 | Virulent |
| T1091 | — | — | 5 | 9 | 9.05 | 9.05 | Virulent |
| T1169 | — | — | 5 | 11 | 17.1 | 17.1 | Virulent |
| T960 | — | — | 7 | 11 | 8.05 | 8.05 | Virulent |
| Mean | — | — | 5 | 11 (Δ = 7) | 15 | 15 | |
| MSP142 | |||||||
| T1139 | 149 | 135 | 7 | 15 | 3.35 | 3.35 | Anemic (V) |
| T1115 | 51 | 136 | 6 | 15 | 4.60 | 4.60 | Virulent |
| T891 | 16 | 73 | 6 | 14 | 10.05 | 10.05 | Virulent |
| T1096 | 37 | 109 | 5 | 28 | 0.07 | 0.00 | Self-cured |
| Mean | 52 | 96,438 | 6 | 18 (Δ = 13) | 5 | 5 | |
| AMA1 | |||||||
| T979 | 311,200 | 168 | — | 28 | 0.00 | 0.00 | Subpatent (Dp) |
| T1089 | 152,600 | 1,975 | 10 | 18 | 0.60 | 0.60 | Anemic (Dp) |
| T1185 | 336,800 | 419 | — | 28 | 0.00 | 0.00 | Subpatent (Dp) |
| T1152 | 87,200 | 7,000 | 6 | 12 | 7.60 | 7.60 | Virulent |
| T1102 | 328,400 | 11,130 | 9 | 21 | 5.10 | 5.10 | Virulent (Dp) |
| T1112 | 82,600 | 1,000 | 22 | 36 | 0.55f | 0.35 | Controlled (Dp) |
| Mean | 183,222 | 1,487 | 17 | 23 (Δ = 7) | 2 | 2 | |
| AMA1 + MSP142 | |||||||
| T1138 | 38,600 | 77,500 | 7 | 14 | 6.80 | 6.80 | Virulent |
| T964 | 161,400 | 87,100 | 24 | 33 | 0.65f | 4.0 | Controlled (Dp) |
| T1173 | 13,850 | 199,100 | 10 | 20 | 0.16 | 0.16 | Anemic (Dp, C) |
| T1188 | 39,000 | 146,600 | 7 | 20 | 0.02 | 0.00 | Anemic (Sc) |
| T914 | 72,200 | 164,400 | 11 | 20 | 5.70 | 5.70 | Virulent (Dp) |
| T1155 | 260,400 | 90,500 | — | 28 | 0.00 | 0.00 | Subpatent (Dp) |
| T986 | 110,975 | 188,700 | — | 14 | 5.60 | 5.60 | Virulent |
| Mean | 61,567 | 108,375 | 13 | 21 (Δ = 9) | 3 | 3 | |
Expressed in arbitrary antibody units, calculated by reference to a standard Aotus serum against the plate antigen. A serum dilution of the reciprocal of the antibody units reported would give an approximate absorbance at 405 nm of 1.0. —, not measured.
Monkeys T979, T1185, and T1155 were subpatent (parasitemia, <0.01%) for the 28-day course of the challenge.
If not already treated, all monkeys were treated on day 28, except T1112 and T964, which were subpatent for 22 and 24 days, respectively, so treatment was deferred in order to monitor the course of their parasitemia. Shown also are the numbers of days between treatment and patency (Δ).
Describes course of infection. Virulent, a sharply rising, uncontrolled parasitemia requiring treatment (parasitemia, >5%); subpatent, no parasites observed per 10,000 RBCs over the course of the challenge; self-cured, parasites cleared by the animal without intervention; controlled, parasite growth controlled to low levels for the 28 days of the study; anemic, monkey required treatment for anemia (hematocrit, <25%). At the time of treatment for anemia, the course of the parasitemia could be virulent (V), controlled (C), or self-cured (Sc). The course of the infection was also modified in some animals by a delay to patency (Dp), where the animal became patent after all control animals had become patent (>7 days).
Shown are geometric mean ELISA titers and arithmetic means for each of the other columns, including only data from the 28 days of challenge. Thus for this purpose, T979, T1185, and T1155 are considered to have 28 days to patency; T1112 and T964 are considered to have 28 days to treatment, with peak parasitemias and parasitemias at day 28 of 0.55 and 0.65, respectively.
Shown is the peak parasitemia within the 28-day challenge period. Monkeys T1112 and T964 were monitored for an additional period, when they eventually had peak parasitemias of 0.75% (T1112) and 4.0% (T964).
The course of the parasitemia for these two control groups illustrates the rigor of this model system, as A. vociferans monkeys are particularly susceptible to P. falciparum infection and in the past have proven more refractory to vaccine-induced protection than some related species (A. nancymai, for example) (15; unpublished data). Additionally, we used the highly virulent P. falciparum FVO parasite clone, resulting in asexual growth rates of 1 order of magnitude every 48 h in naïve monkeys (Fig. 1A), close to the theoretical maximum for P. falciparum (and that observed with naïve human volunteers) (4).
Protection from P. falciparum induced by vaccination with bvMSP142.
As shown in Fig. 1C, vaccination with bvMSP142 did confer significant protection (P = 0.03 for comparison to the Pvs25H group, and P = 0.006 for comparison to the combined unvaccinated and Pvs25H groups, by the Mann-Whitney U test). Of the four animals in this group, one animal controlled its parasitemia (<0.07%) before self-curing and although a second animal required treatment for anemia rather than parasitemia, it appeared to have lost control of its parasitemia at this point (T1139). The remaining two animals also suffered virulent infections (Fig. 1C; Table 1). The protection seen in the bvMSP142 group was a result of slowing the parasite's rate of growth rather than preventing infection (the mean number of days from patency to treatment for the bvMSP142 group was 13, compared to 7 for both the Pvs25H and naïve groups [Table 1]). Thus, the prepatency period was not affected by vaccination with bvMSP142 (P = 1.0 for comparison of days to patency [≥0.01% parasitemia] with the Pvs25H group, and P = 0.57 for comparison with all nine controls, by the Mann-Whitney U test); the growth rates, however, were significantly slowed (P = 0.036 for comparison of days of patent parasitemia in animals with virulent infections by the Mann Whitney U test; P = 0.009 for comparison with all nine controls). This is consistent with the findings of our previous studies with bvMSP142, where we did not generally see an increased prepatent period.
Protection from P. falciparum induced by vaccination with AMA1.
Vaccination with AMA1 conferred significant protection (Fig. 1B; Table 1). In comparison with the Pvs25H group, the AMA1 monkeys had significantly fewer parasites (P = 0.009 by the Mann-Whitney U test), as they did in comparison to all controls (P = 0.0008 by the Mann-Whitney U test). Two of the six animals in this group had peak parasitemias that were subpatent, or below 0.01%, for the 28-day challenge period. In addition, monkey T1112 maintained a subpatent parasitemia for 22 days, after which it controlled its parasitemia for a further 14 days at <0.75% before requiring treatment for anemia at 0.35% parasitemia. One additional animal required treatment for anemia while controlling its parasitemia at <0.6% (T1089), and two animals suffered acute parasitemias, although one had a delayed onset (T1102 received treatment on day 21 for 5.1% parasitemia, compared to a mean of day 11.6 for the Pvs25H group).
Table 1 shows that for the AMA1-alone group, the prepatent period is significantly increased over that for the Pvs25H-vaccinated group (P = 0.017 for comparison of days to patency [≥0.01% parasitemia] with the Pvs25H group; P = 0.0028 for comparison with all nine controls). Growth rates are available for only two animals in the AMA1 group (only T1152 and T1102 suffered acute virulent infections requiring treatment for parasitemia), and these are not significantly different from the Pvs25H group's growth rates (P = 0.38).
Protection from P. falciparum induced by vaccination with the combination of AMA1 plus bvMSP142.
Vaccination with the AMA1-plus-bvMSP142 combination also conferred significant protection (P = 0.003 for comparison with the Pvs25H group, and P = 0.0002 for comparison with all nine controls, by the Mann-Whitney U test). In this group, one animal had a subpatent infection, two animals required treatment for anemia while controlling or curing their infections (T1173 and T1188), and one animal (T964) maintained a subpatent parasitemia for 24 days, requiring treatment for anemia 9 days after the infection reached patency. One of the remaining animals (T914) had an extended prepatency period before eventually succumbing to acute parasitemia along with T1138 and T986.
Only in this combination group was there both a significant delay in patency (P = 0.013 for comparison to the Pvs25H group; P = 0.0024 for comparison with all nine controls) and a significant decrease in growth rates (P = 0.032 for comparison to the Pvs25H group; P = 0.0084 for comparison with all nine controls).
Antibody responses induced by vaccination.
Antibody titers to AMA1 in animals that received AMA1 either alone or in combination with MSP142 correlated with protection as measured by days to patency (P = 0.0074; r2 = 0.47), days to treatment (P = 0.015; r2 = 0.36), or peak parasitemia (P = 0.003; r2 = 0.52). Examined in individual groups, the correlations were strong enough to reach significance only for the animals receiving AMA1 plus bvMSP142 (P = 0.0012 and r2 = 0.86, P = 0.013 and r2 = 0.65, and P = 0.013 and r2 = 0.66, respectively). Antibody titers to bvMSP142, on the other hand, did not correlate with protection (either among all monkeys or in the individual bvMSP142-alone and AMA1-plus-bvMSP142 groups).
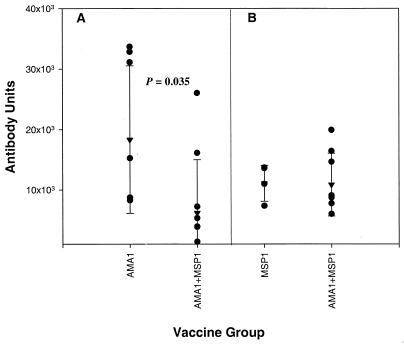
Vaccination with AMA1 plus bvMSP142 resulted in a significant decrease in titers of antibodies to AMA1 (geometric mean AMA1 titers, 183,222 in AMA1-vaccinated animals and 61,567 in combination-vaccinated animals; P = 0.035 by the Mann-Whitney U test) (Fig. 2). No decrease in MSP142 antibody levels was observed in the AMA1-plus-bvMSP142 group relative to the bvMSP142-alone group. Indeed, of the seven animals in the AMA1-plus-bvMSP142 vaccination group, only two had antibody levels above the minimum observed antibody level in the AMA1-alone group. These were the two best-protected animals (T964 and T1115), and it is reasonable to conclude that anti-AMA1 antibodies played a major part in the protective efficacy. Further evidence of this is the delay in patency for these two animals, consistent with the type of protection elicited in the AMA1-alone group, where patency was significantly delayed. Since the remaining five animals had antibody levels below the minimum seen in the AMA1-alone group, it is reasonable to conclude that any protective efficacy may have been due largely to responses against both antigens, and indeed two of the five showed delays in patency (T1173 and T914) and four of the five had slower growth rates (T1173, T1188, T914, and T986).
FIG. 2.
Reciprocal antibody titers in vaccinated monkeys. (A) Antibody titers to AMA1 in monkeys receiving AMA1either alone or in combination with MSP142. (B) Antibody titers to MSP142 in monkeys receiving MSP142 either alone or in combination with AMA1. Shown are individual titers (•), geometric mean titers (▾), and standard deviations (error bars). ELISA titers are expressed in arbitrary antibody units, calculated by reference to a standard Aotus serum against the plate antigen. A serum dilution of the reciprocal of the antibody units reported would give an approximate absorbance at 405 nm of 1.0.
In agreement with the results from rabbit immunizations (13a), the antibody elicited by vaccination of Aotus monkeys with AMA1 also showed allele specificity. Among all 13 animals receiving AMA1, the geometric mean of the ratios of the antibody responses (as measured by ELISA) to the FVO allele of AMA1 to the antibody responses to the 3D7 allele of AMA1 was 1.9.
Although the combination of AMA1 plus MSP142 reduced anti-AMA1 titers, there was no evidence that the specificity of the response was altered, since the FVO/3D7 ELISA titer ratios in the AMA1-alone group and the AMA1-plus-MSP142 group were not significantly different (geometric mean ratios, 1.8 and 2.0, respectively; P = 0.2).
In cases where protection was associated with a delayed prepatency period, the possibility of infections that escaped protection were of concern. There was no evidence of antibody consumption leading to the late escape of parasites (in T1102, T1112, T914, or T964, for example); in fact, antibody levels remained constant during the challenge period (data not shown). The AMA1 genes from these parasites were sequenced, and the sequences remained unchanged from that of the original infectious isolate.
DISCUSSION
Vaccination with bvMSP142 gave significant protection from an otherwise lethal challenge with P. falciparum FVO parasites. The nature of this protective efficacy was to slow parasite growth rates significantly compared to those for control animals, but this was not sufficient to increase the prepatency period or allow more than one animal out of four to self-resolve the infection.
This result is less protective than those obtained with bvMSP142 in A. nancymai monkeys in three previous studies: two of seven self-curing (clearing the infection) and four of seven controlling virulent parasite growth to below treatment levels but requiring treatment for anemia (18); one of seven self-curing and four of seven controlling parasitemia but requiring treatment for anemia (10); and four of seven self-curing and one of seven controlling parasitemia but requiring treatment for anemia (17). However, the present result was not unexpected in this virulent challenge model (A vociferans monkeys challenged with P. falciparum FVO parasites). In a previous study comparing A. vociferans and A. nancymai monkeys by using a form of MSP1 (MSP119) as the vaccine, the A. nancymai monkeys (two animals) were highly protected, while the A. vociferans monkeys (two animals) had virulent infections (15). Similarly, in a later trial using the same vaccine, again comparing A. vociferans and A. nancymai monkeys, the A. nancymai monkeys (two) were highly protected, while one A. vociferans monkey had a virulent infection and the second required treatment for anemia with a parasitemia of 1.0% (14). Again, in an unpublished study in our lab, of seven A. vociferans monkeys vaccinated with MSP119, five required treatment for uncontrolled parasitemia while one self-resolved and another experienced a chronic infection (A. W. Stowers, unpublished data).
Given this history, the protection achieved by vaccinating A. vociferans monkeys with recombinant P. falciparum AMA1 is striking. Two of six monkeys, or 33%, had subpatent parasitemias (i.e., <0.01% infected RBCs) for the entire 28-day course of the infection. A third monkey was subpatent for 22 days (compared with an average of 6 days for control animals).
The challenge data also suggest that the mechanisms of protection may be different for AMA1 and MSP142. Table 1 shows that the prepatency period is significantly longer for the AMA1-alone group than for the Pvs25H-vaccinated group (P = 0.017). For the bvMSP142 group, no such increase in patency was observed; rather, these animals showed a decrease in growth rates that was not observed for the animals that suffered virulent infections in the AMA1 group.
This is the first time we have observed a significant delay in patency as a result of vaccination. In eight published studies (and three unpublished studies by Stowers and coworkers) with A. nancymai or A. vociferans monkeys and MSP1-based vaccines (8, 9, 14, 15, 17, 18), an MSP3 vaccine (10), or whole parasites (13), no significant delay in patency compared to that for control animals has been observed. The majority of these studies were performed with A. nancymai, so it is possible that a delay in patency is a characteristic of the A. vociferans model. Indeed, in the three A. vociferans studies described above, 5 of 12 animals did have delayed patencies. We do not believe this to be an artifact of the model, though, because in the present study, five of the six AMA1-vaccinated animals had delayed patencies, compared with none of the bvMSP142-vaccinated animals, even though bvMSP142 vaccination clearly had some protective efficacy.
Of concern is the possibility of infections that escaped protection. There was no evidence of antibody consumption leading to the late escape of parasites (in T1102, T1112, or T964, for example), and the sequences of the AMA1 genes from these parasites remained unchanged from that of the original infectious isolate. It is possible that protection was due to a subset of high-affinity antibodies or that a selection for parasites utilizing an AMA1-independent invasion pathway occurred with or without a loss of AMA1 gene expression. These parasites were subsequently lost, and so it was not possible to confirm AMA1 expression. It is also possible that a vaccine-induced antibody to AMA1 allowed most monkeys to control early parasite growth but that other cellular mechanisms of immunity, which not all monkeys were successful in making, were required for clearance. The role of cytokines, for example, in both protection and pathogenesis in malaria is well known (19). Successful deployment of a malaria vaccine may require targeting of these mechanisms as well as individual antigens (21). Whether our results are an anomaly of the Aotus model (with nonnative ligands on the Aotus RBCs) will not be answered until the first human phase II field trials, several years away.
The results from our AMA1-plus-bvMSP142 combination vaccine were mixed. Although the combination did not improve treatment outcomes over those for vaccination with AMA1 alone, it may have altered parasitological outcomes. Only in the combination group was there both a significant delay in patency (P = 0.013) and a significant decrease in growth rates (P = 0.032). This is evidence that responses to both antigens, operating through slightly different mechanisms, were elicited.
However, the protection elicited by the combination vaccination was certainly not significantly better than that elicited by vaccination with AMA1 alone. Only the two best-protected animals in the combination group had antibodies above the minimum observed antibody level in the AMA1-alone group. And overall, vaccination with both AMA1 and bvMSP142 resulted in a significant decrease in titers of antibodies to AMA1 (P = 0.035), while no decrease was observed for MSP142 antibodies. This suggests that the combination may have had an overall detrimental effect in comparison with vaccination with AMA1 alone, and it highlights the need to proceed carefully with combination vaccines.
To our knowledge, this form of recombinant P. falciparum AMA1 and its combination with MSP142 have proved to be the most efficacious vaccines ever tested in Aotus monkeys, especially given the rigorous model system of A. vociferans and P. falciparum FVO parasites. In areas of endemicity, children may carry significant parasitemias asympomatically for extended periods. Thus, based on the AMA1-vaccinated group of monkeys, where two of six animals remained subpatent for the course of the infection and two controlled parasite growth below 0.75%, it is feasible that a properly formulated asexual vaccine based on AMA1 could induce asymptomatic protection. These data provide a solid basis for testing this form of recombinant AMA1 in humans in an appropriate adjuvant formulation. Combinations of AMA1 with MSP142 may elicit broader immune response mechanisms than immunization with AMA1 alone.
Acknowledgments
We thank Lynn Lambert and Joshua Reece for animal husbandry, and Farideh Chitsaz for sequencing the ama1 gene from the escape parasites. We also thank Robin Anders and Alan Thomas for very helpful discussions.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Amante, F. H., P. E. Crewther, R. F. Anders, and M. F. Good. 1997. A cryptic T cell epitope on the apical membrane antigen 1 of Plasmodium chabaudi adami can prime for an anamnestic antibody response: implications for malaria vaccine design. J. Immunol. 159:5535-5544. [PubMed] [Google Scholar]
- 2.Anders, R. F., P. E. Crewther, S. Edwards, M. Margetts, M. L. Matthew, B. Pollock, and D. Pye. 1998. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 16:240-247. [DOI] [PubMed] [Google Scholar]
- 3.Chang, S. P., S. E. Case, W. L. Gosnell, A. Hashimoto, K. J. Kramer, L. Q. Tam, C. Q. Hashiro, C. M. Nikaido, H. L. Gibson, C. T. Lee-Ng, P. J. Barr, B. T. Yokota, and G. S. Hui. 1996. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect. Immun. 64:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, Q., G. Lawrence, C. Reed, A. Stowers, L. Ranford-Cartwright, A. Creasey, R. Carter, and A. Saul. 1997. Measurement of Plasmodium falciparum growth rates in vivo: a test of malaria vaccines. Am. J. Trop. Med. Hyg. 57:495-500. [DOI] [PubMed] [Google Scholar]
- 5.Collins, W. E., D. Pye, P. E. Crewther, K. L. Vandenberg, G. G. Galland, A. J. Sulzer, D. J. Kemp, S. J. Edwards, R. L. Coppel, J. S. Sullivan, et al. 1994. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am. J. Trop. Med. Hyg. 51:711-719. [DOI] [PubMed] [Google Scholar]
- 6.Crewther, P. E., M. L. Matthew, R. H. Flegg, and R. F. Anders. 1996. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect. Immun. 64:3310-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deans, J. A., A. M. Knight, W. C. Jean, A. P. Waters, S. Cohen, and G. H. Mitchell. 1988. Vaccination trials in rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66 kD merozoite antigen. Parasite Immunol. 10:535-552. [DOI] [PubMed] [Google Scholar]
- 8.Egan, A. F., M. J. Blackman, and D. C. Kaslow. 2000. Vaccine efficacy of recombinant Plasmodium falciparum merozoite surface protein 1 in malaria-naïve, -exposed, and/or -rechallenged Aotus vociferans monkeys. Infect. Immun. 68:1418-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrera, M. A., F. Rosero, S. Herrera, P. Caspers, D. Rotmann, F. Sinigaglia, and U. Certa. 1992. Protection against malaria in Aotus monkeys immunized with a recombinant blood-stage antigen fused to a universal T-cell epitope: correlation of serum gamma interferon levels with protection. Infect. Immun. 60:154-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hisaeda, H., A. Saul, J. J. Reece, M. C. Kennedy, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. Merozoite surface protein 3 and protection against malaria in Aotus nancymai monkeys. J. Infect. Dis. 185:657-664. [DOI] [PubMed] [Google Scholar]
- 11.Hisaeda, H., A. W. Stowers, T. Tsuboi, W. E. Collins, J. S. Sattabongkot, N. Suwanabun, M. Torii, and D. C. Kaslow. 2000. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect. Immun. 68:6618-6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodder, A. N., P. E. Crewther, and R. F. Anders. 2001. Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. 69:3286-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones, T. R., N. Obaldia III, R. A. Gramzinski, and S. L. Hoffman. 2000. Repeated infection of Aotus monkeys with Plasmodium falciparum induces protection against subsequent challenge with homologous and heterologous strains of parasite. Am. J. Trop. Med. Hyg. 62:675-680. [DOI] [PubMed] [Google Scholar]
- 13a.Kennedy, M. C., J. Wang, Y. Zhang, A. P. Miles, F. Chitsaz, A. Saul, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect. Immun. 70:6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar, S., W. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. Guevara Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 68:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar, S., A. Yadava, D. B. Keister, J. H. Tian, M. Ohl, K. A. Perdue-Greenfield, L. H. Miller, and D. C. Kaslow. 1995. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol. Med. 1:325-332. [PMC free article] [PubMed] [Google Scholar]
- 16.Narum, D. L., S. A. Ogun, A. W. Thomas, and A. A. Holder. 2000. Immunization with parasite-derived apical membrane antigen 1 or passive immunization with a specific monoclonal antibody protects BALB/c mice against lethal Plasmodium yoelii yoelii YM blood-stage infection. Infect. Immun. 68:2899-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stowers, A. W., L. H. Chen, Y. Zhang, M. C. Kennedy, L. Zou, L. Lambert, T. J. Rice, D. C. Kaslow, A. Saul, C. A. Long, H. Meade, and L. H. Miller. 2002. A recombinant vaccine expressed in the milk of transgenic mice protects Aotus monkeys from a lethal challenge with Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 99:339-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stowers, A. W., V. Cioce, R. L. Shimp, M. Lawson, G. Hui, O. Muratova, D. C. Kaslow, R. Robinson, C. A. Long, and L. H. Miller. 2001. Efficacy of two alternate vaccines based on Plasmodium falciparum merozoite surface protein 1 in an Aotus challenge trial. Infect. Immun. 69:1536-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor-Robinson, A. W., and E. C. Smith. 1999. A role for cytokines in potentiation of malaria vaccines through immunological modulation of blood stage infection. Immunol. Rev. 171:105-123. [DOI] [PubMed] [Google Scholar]
- 20.Xu, H., A. N. Hodder, H. Yan, P. E. Crewther, R. F. Anders, and M. F. Good. 2000. CD4+ T cells acting independently of antibody contribute to protective immunity to Plasmodium chabaudi infection after apical membrane antigen 1 immunization. J. Immunol. 165:389-396. [DOI] [PubMed] [Google Scholar]
- 21.Xu, H., J. Wipasa, H. Yan, M. Zeng, M. O. Makobongo, F. D. Finkelman, A. Kelso, and M. F. Good. 2002. The mechanism and significance of deletion of parasite-specific CD4+ T cells in malaria infection. J. Exp. Med. 195:881-892. [DOI] [PMC free article] [PubMed] [Google Scholar]